(UroToday.com) The 2024 Bladder Cancer Advocacy Network (BCAN) Bladder Cancer Think Tank held in San Diego, CA was host to a BCAN 2023 Patient Centered Clinical Young Investigator Awardee session. Dr. Rishi Sekar, the Patient-Centered Clinical Research Young Investigator Awardee, discussed his work evaluating individual and community-level drivers of disparities in bladder cancer clinical trials participation.
Dr. Sekar noted that cancer, which remains prevalent in our society, has a major impact on patients’ quantity and quality of life. It is also a source of significant economic burden, both for the patient and society as a whole. Clinical trials, and the resultant scientific findings, have advanced the practice of oncology leading to novel screening and diagnostic approaches, therapeutics and interventions, and, thus, standards of care for our patients. The ultimate aim is to save, extend, and improve patient lives.
The NCCN currently notes that the ‘best management for any patient with cancer is in a clinical trial. Participation in clinical trials is especially encouraged. Participation in a clinical trial has been demonstrated to ensure high quality care and improve cancer outcomes in some studies. Clinical trials offer participants novel treatment approaches that may not yet be approved in clinical practice. Furthermore, these patients classically undergo regimented follow-ups that minimize care deficiencies. Thus, Dr. Sekar argued that the opportunity to participate in a clinical trial should be available to all patients, particularly those with oncologic diseases.
However, only 5–7% of eligible patients participate in trials, despite ~50% demonstrating a willingness to participate, if presented with the opportunity. Minority populations remain severely underrepresented despite having worse cancer outcomes, exacerbating known disparities. Black and Hispanic patients comprise <5% of bladder cancer clinical trial participants, despite accounting for a significantly higher proportion of the disease population. Females with bladder cancer are also underrepresented in bladder cancer clinical trials despite typically having a more aggressive disease at presentation. Similar patterns are also seen when looking at adverse social determinants of health, including underinsurance, poverty, and rurality. The ensuing consequence of underrepresentation include:

Social determinants of health are non-medical variables that impact the receipt of medical care and have been shown to have a greater influence on health compared to genetic factors. Many barriers to clinical trial participation are ‘rooted’ in social determinants of health and include:
- Travel issues
- Cost
- Family/household support
- Culture/language
- Built environment

How do we measure social determinants of health? It can be measured at the individual level by asking patients about their income, education, family, transportation, neighborhood, etc. That is the most granular method; however, this is time consuming and challenging to perform on a large scale. Conversely, this can be measured at the population level, by linking patients to aggregated measures of the neighborhood, county, or state they live in. While this is a simpler method, it does come with the limitations of internal heterogeneity. In his work, Dr. Sekar chose to use a population-level measure of social determinants of health developed by the CDC: The Social Vulnerability Index. This is a county-level measure of vulnerability that is derived from the American Community Survey using 15 social variables and includes themes of socioeconomic status, household characteristics, racial and ethnic composition, and housing type and transportation.
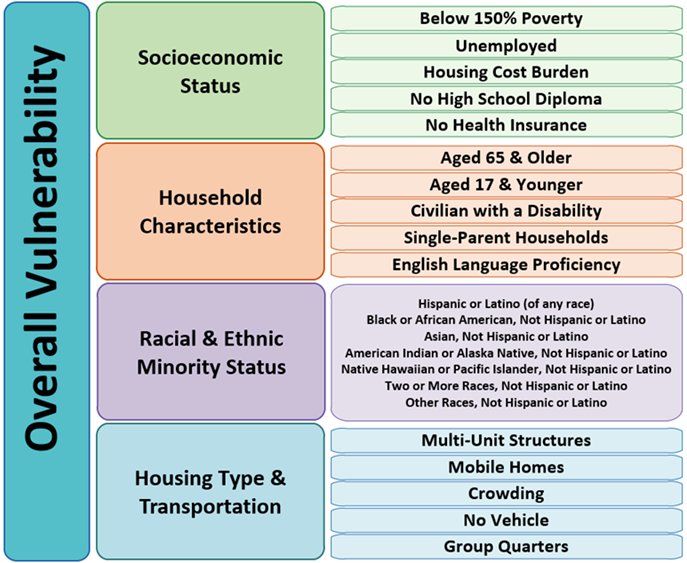
The aims of Dr. Sekar’s work in this field include the following:
- To understand the association of patient demographics and community-level social determinants of health on clinical trials awareness, perceptions, and participation
- To characterize the geographic variations in clinical trial availability and the association with community-level social determinants of health and bladder cancer mortality
Dr. Sekar and his team first sought to understand:
- Levels of knowledge of clinical trials
- Who is being offered clinical trial opportunities
- Who is deciding to participate
Accordingly, they utilized a novel representative survey conducted by the National Cancer Institute, HINTS-SEER, which surveyed 1,234 cancer survivors representing a population of >400,000 patients of all cancer types. The primary study exposure was the social vulnerability index, which is a county level measure of social determinants of health. Additional exposures assessed included individual-level demographics, education, income, and cancer stage.
Their outcomes of interest included the following patient-reported variables:
- How would you describe your level of knowledge of clinical trials?
- Has your medical team discussed clinical trials as a possible treatment option for your cancer?
- Have you participated in a clinical trial for the treatment of your cancer?
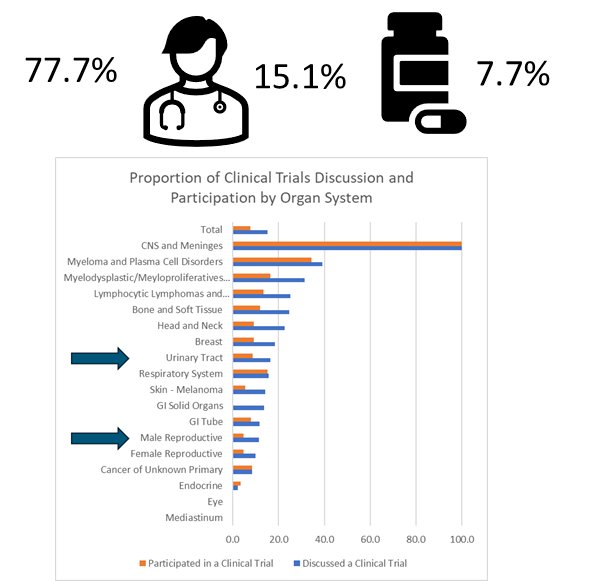
Overall, only 15.1% of patients were aware of clinical trials, and only 7.7% participated in a clinical trial.
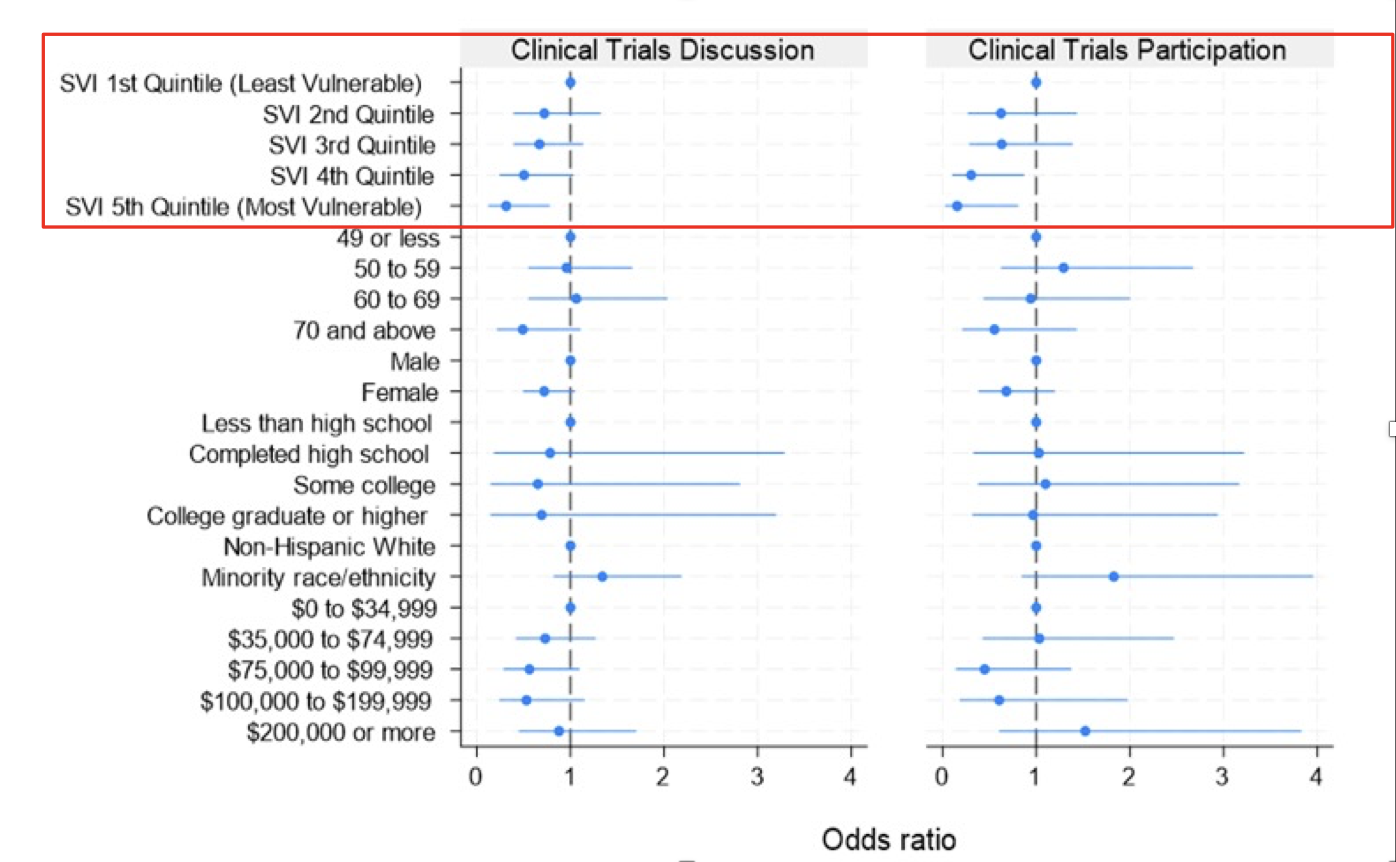
The most socially vulnerable patients had lower:
- Awareness of clinical trials (8.5% versus 17.9% for least socially vulnerable)
- Participation rates (3% versus 10.1% for the least socially vulnerable)
Furthermore, there appeared to be a ‘dose-effect’ relationship whereby increasing social vulnerability was associated with stepwise lower odds of trial discussion and participation. Notably, no other variable, including income and education, was significantly associated with the outcomes of trial discussion and participation.
The key takeaway from this work is that where patients live matters, and the characteristics of their community impact trial awareness, discussion, and participation.
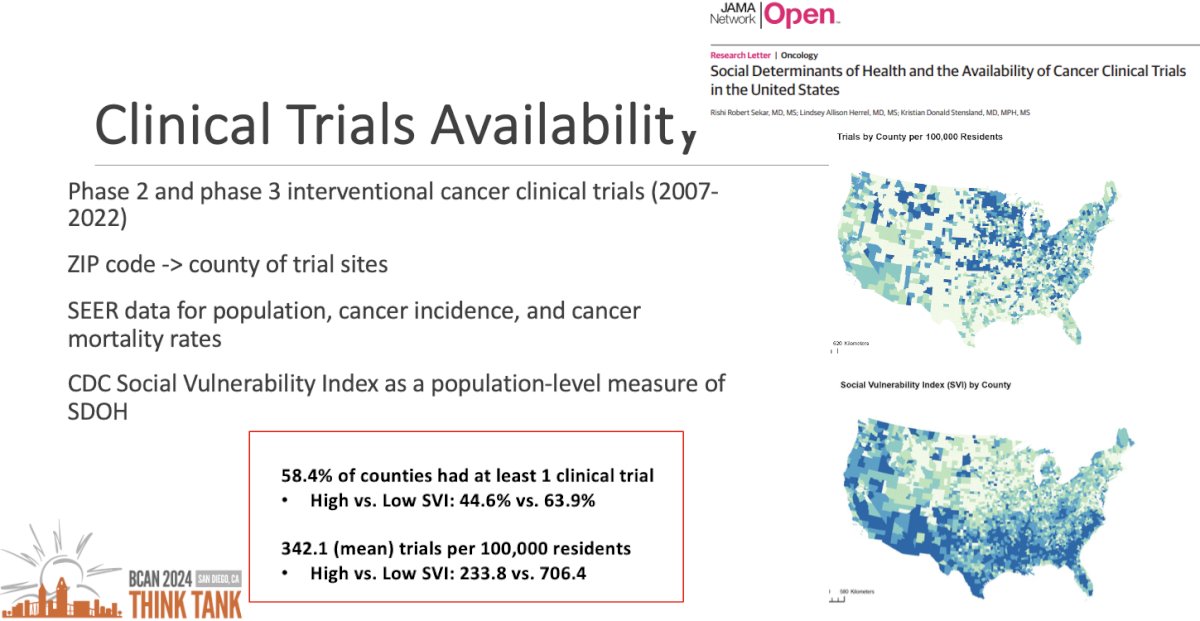
The next objective was to characterize the geographic variations in clinical trial availability and association with community-level social determinants of health. Dr. Sekar and colleagues performed an ecological study of cancer clinical trial opportunities throughout the United States.1 They included phase II and III interventional cancer clinical trials for the most common cancers between 2007 and 2022. For each county, they assessed if any trials were available, and, if yes, how many. They compared this across social vulnerability strata, adjusting for various factors like population, cancer incidence, and presence of cancer hospitals. They demonstrated that:
- 58.4% of counties had ≥1 clinical trial
- High versus low social vulnerability index: 44.6% versus 63.9%
- There was a mean of 342.1 trials per 100,000 residents
- High versus low social vulnerability index: 233.8 versus 706.4 trials
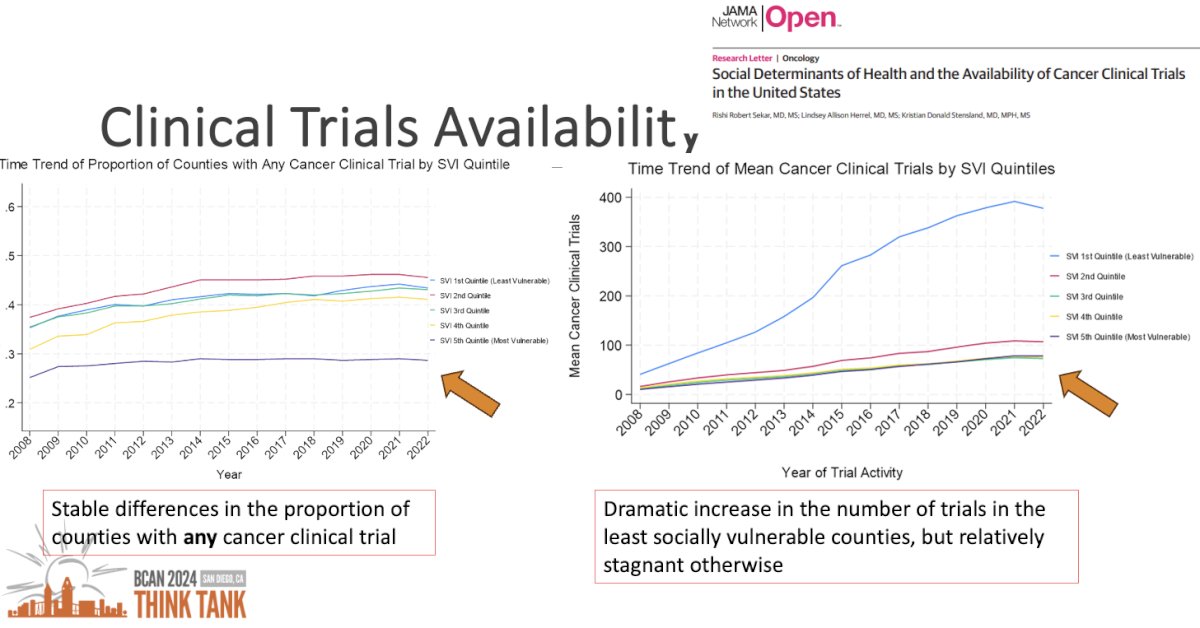
Evaluating trends over time, the investigators noted that, overall, the proportion of counties with any clinical trial available, stratified by social variability index quintile, remained stable over time. However, there was a dramatic increase in the absolute number of trials available in the least socially vulnerable counties, as demonstrated below.
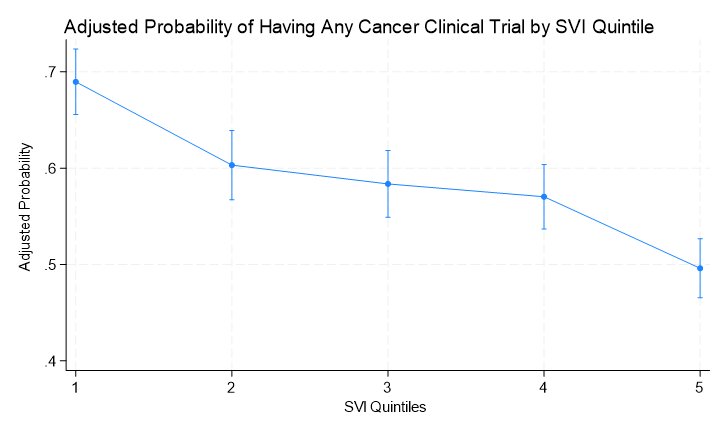
Similar trends were observed on adjusted analysis accounting for population size, cancer incidence rates, and presence of cancer hospitals. With increasing social vulnerability, there was a lower probability of having any clinical trial, with a ~20% difference between the least and most vulnerable counties.
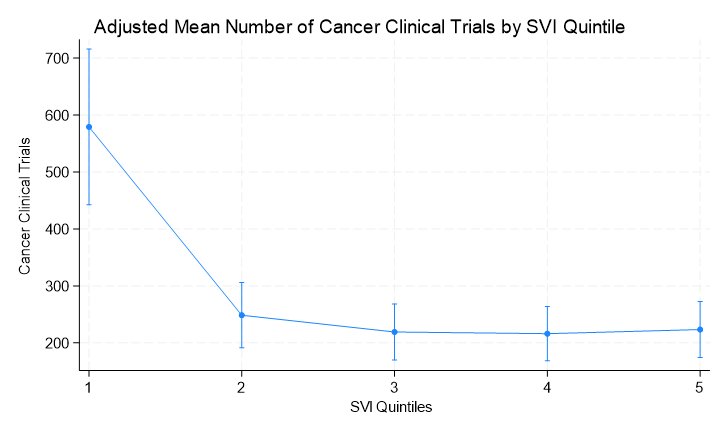
A similar trend was observed when evaluating the adjusted mean number of clinical trials by social vulnerability index quintile.
Are trial opportunities available to communities that may benefit the most? In other words, are clinical trials available in those counties with the highest bladder cancer mortality rates? In summary, the most socially vulnerable counties are far less likely to have any bladder cancer clinical trials, despite having higher bladder cancer mortality rates. This is illustrated in the image below: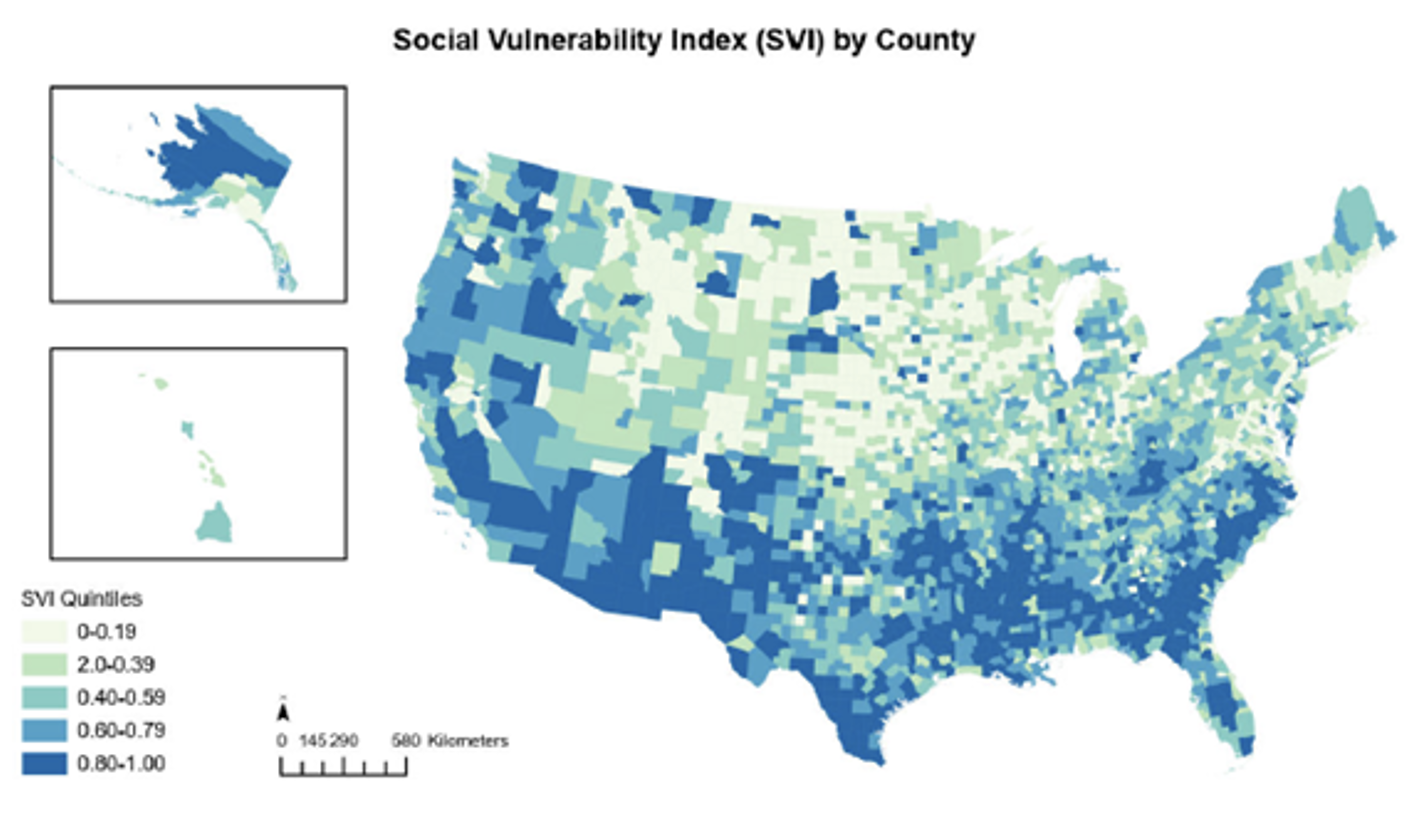
Social vulnerability map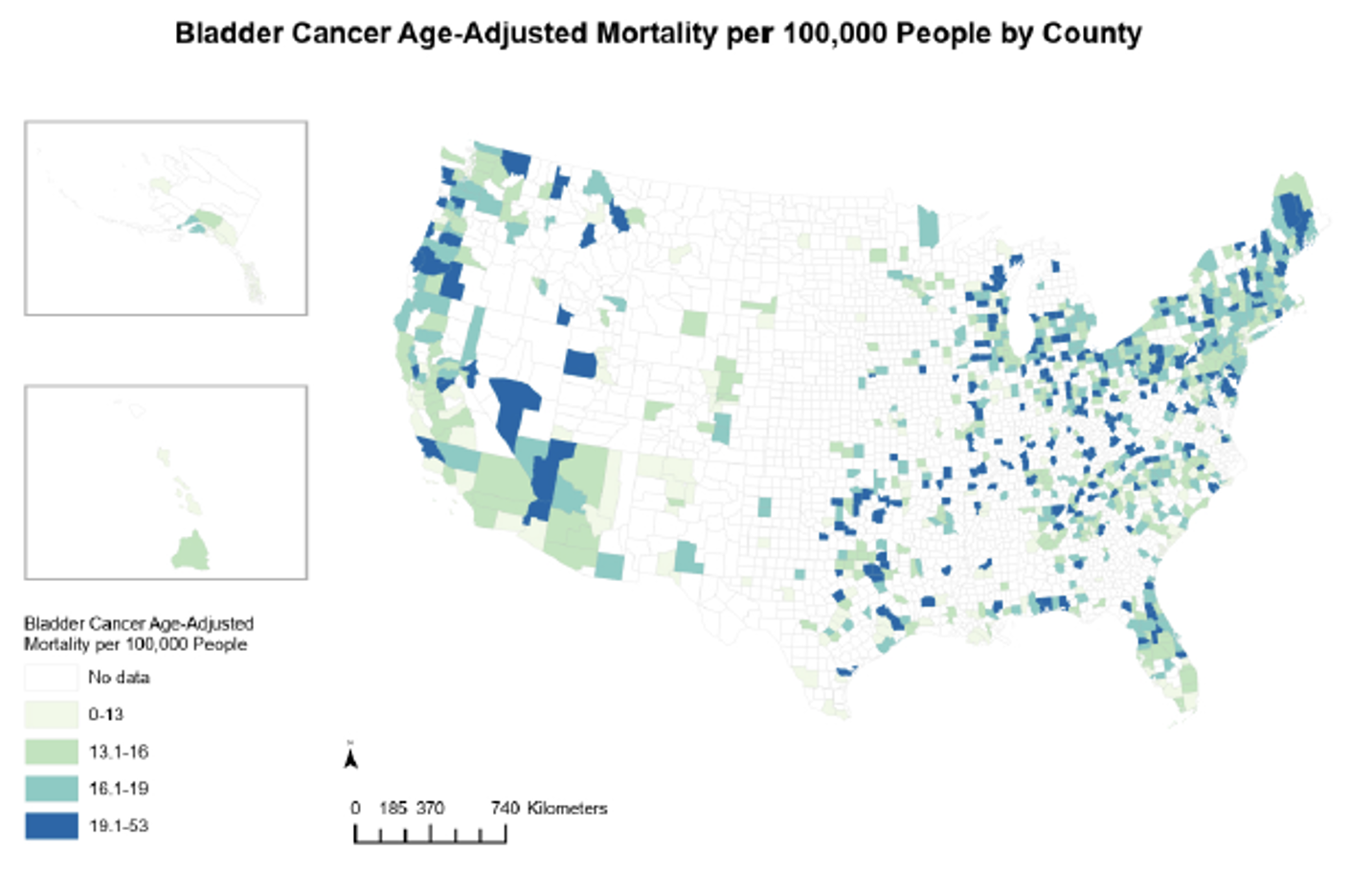
Choropleth map of bladder cancer mortality rates per 100,000 residents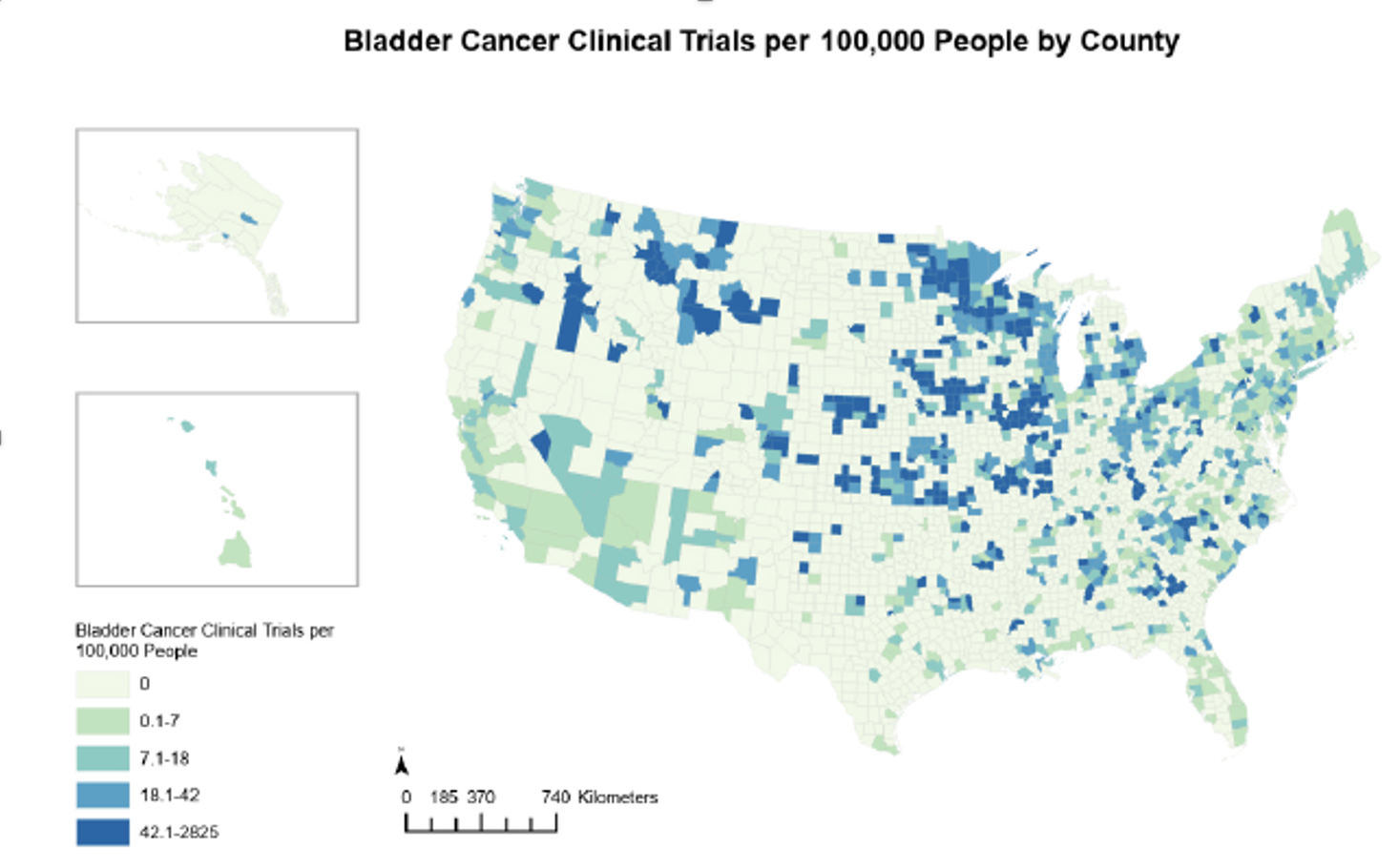
Number of bladder cancer trials by county
Furthermore, Dr. Sekar’s team demonstrated that:
- Bladder cancer mortality significantly increases with community social vulnerability
- The probability of having any trial, and the number of trials, significantly decreases with increasing community social vulnerability
Based on this work, Dr. Sekar concluded that:
- Socially vulnerable patients, or those facing adverse community-level social determinants of health, are less likely to discuss clinical trials as a treatment option, participate in a trial, or have trial opportunities within their community
- More diverse, socioeconomically disadvantaged communities experience adverse cancer outcomes
- The characteristics of the community might matter more than those of the individual
- These findings support efforts for the expansion of clinical trial infrastructure and opportunities into socially vulnerable communities to improve representation and enhance equitable cancer care
Presented by: Rishi Sekar, MD, Society of Urologic Oncology Fellow, Department of Urology, University of Michigan, Ann Arbor, MI
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 BCAN Bladder Cancer Think Tank held in San Diego, CA between August 7th and 9th, 2024
Reference:
- Sekar RR, Herrel LA, Stensland KD, et al. Social Determinants of Health and the Availability of Cancer Clinical Trials in the United States. JAMA Netw Open. 2024;7(5):e2410162.


