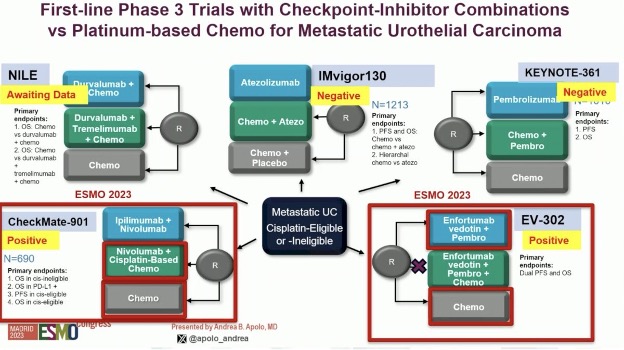
Prior to ESMO 2023, where EV-302 and CheckMate 901 were both first presented, the standard of care management of advanced/metastatic urothelial carcinoma was significantly different, whereby platinum-based combination chemotherapy (gem/cis or ddMVAC) was considered 1st line therapy, patients who were platinum-ineligible were recommended for pembrolizumab monotherapy, and enfortumab vedotin was recommended in ≥3rd line setting, although it did receive accelerated approval for cisplatin-ineligible patients prior to ESMO 2023.

Enfortumab vedotin is an antibody-drug conjugate targeting Nectin-4, which is a tumor-associated antigen found on the surface of most urothelial carcinoma cells. Its cell surface expression decreases as urothelial carcinoma progresses through more advanced stages, providing a strong rationale for the use of enfortumab vedotin in the front-line setting.
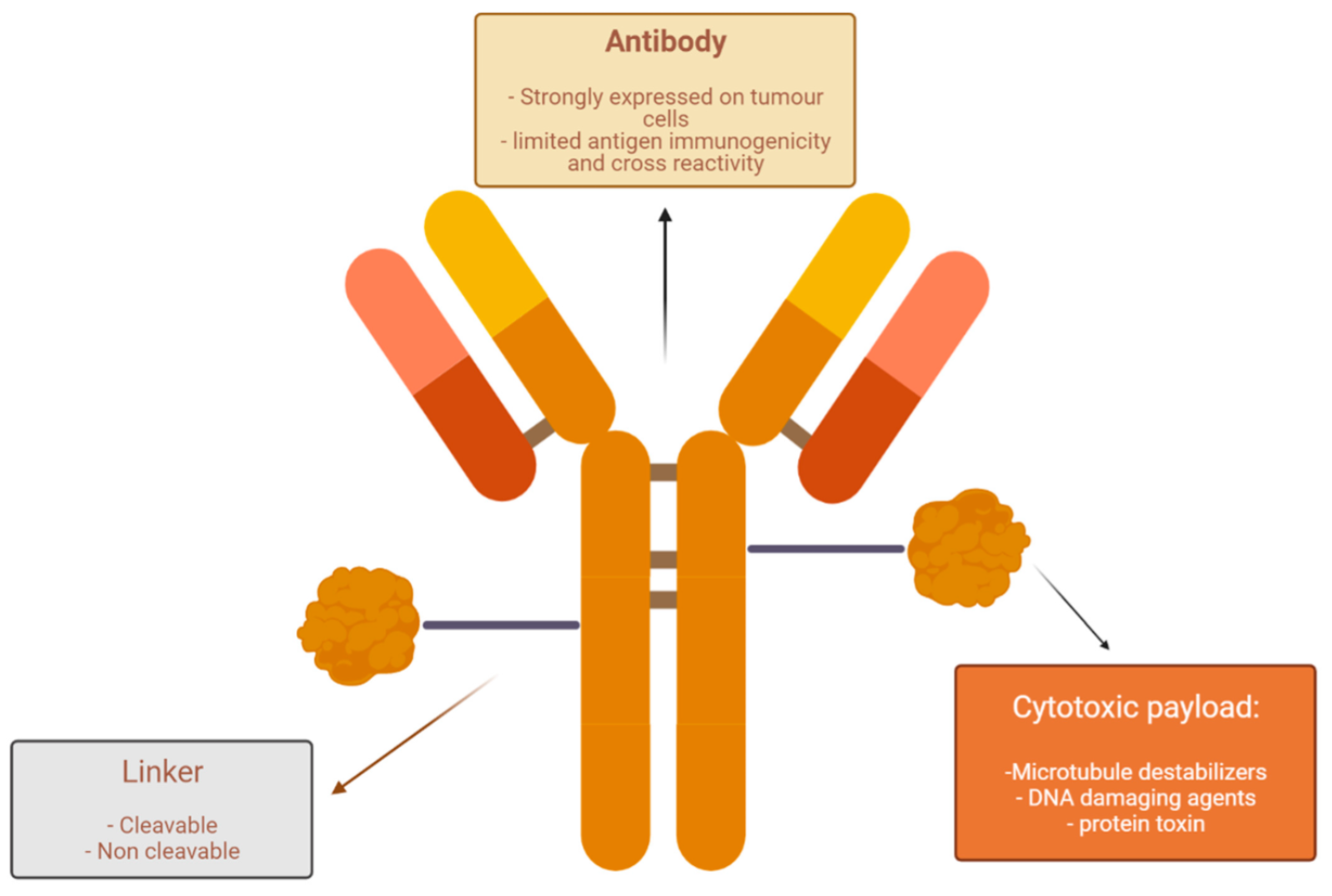
Enfortumab vedotin is made of three parts:
- The antibody: Anti-Nectin-4
- The payload: MMAE
- The linker (stable in circulation, but releases the cytotoxic agent in the target cell)
Nectin-4 is highly expressed in metastatic urothelial cancer patients, which does not necessitate tumor screening. The payload MMAE (plus linker) is vedotin, a microtubule-disrupting agent (200× more potent than vinblastine).
In December 2019, the FDA granted accelerated approval of enfortumab vedotin for two indications:
- Platinum and PD-1/PD-L1 refractory metastatic urothelial carcinoma
- Cisplatin-ineligible patients who had previously received PD-1/PD-L1 therapy
Discussing EV-302 first, she noted that this trial randomized 442 patients, irrespective of cisplatin eligibility and PD-L1 expression status, in a 1:1 fashion to either enfortumab vedotin + pembrolizumab (n=442) or gemcitabine + cisplatin/carboplatin (n=444) for a maximum of 6 cycles. Notably, there were no maximum treatment cycles for enfortumab vedotin (i.e., continued until clinical/disease progression or unacceptable toxicity), whereas pembrolizumab was administered for a maximum of 35 cycles. The dual primary endpoints were progression-free survival, assessed via blinded independent central review (BICR), and overall survival. The study design is as follows:

Approximately 25% of patients had upper tract disease. Overall, 46% of patients were cisplatin-ineligible, and 58% had tumors with a high PD-L1 expression. Liver metastases, a known adverse prognostic factor, were present in 22% of patients.
Compared to platinum-based chemotherapy, enfortumab vedotin + pembrolizumab prolonged progression-free survival from a median of 6.3 months to 12.5 months (HR: 0.45, 95% CI: 0.38 – 0.45, p<0.001):

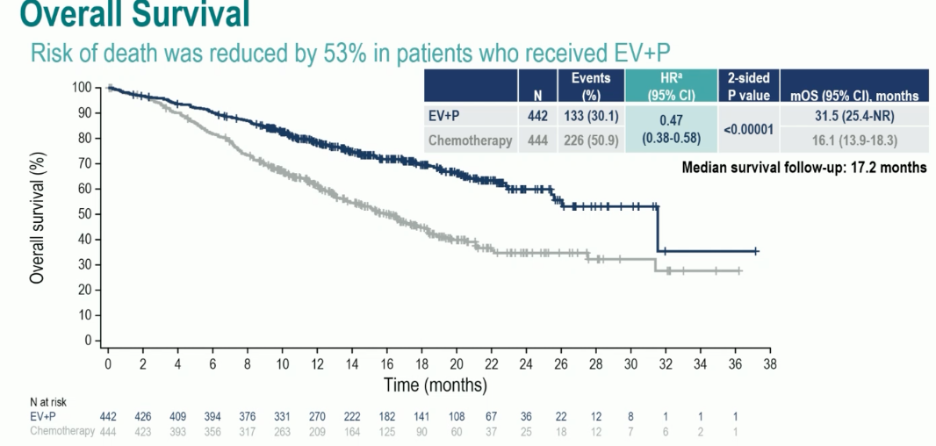
Overall survival was nearly doubled in the enfortumab vedotin + pembrolizumab arm, with median survivals of 31.5 and 16.1 months, respectively (HR: 0.47, 95% CI: 0.38 – 0.58, p<0.00001). These survival benefits were observed despite a higher proportion of patients in the chemotherapy arm receiving subsequent systemic therapy (66.2% versus 28.9%):
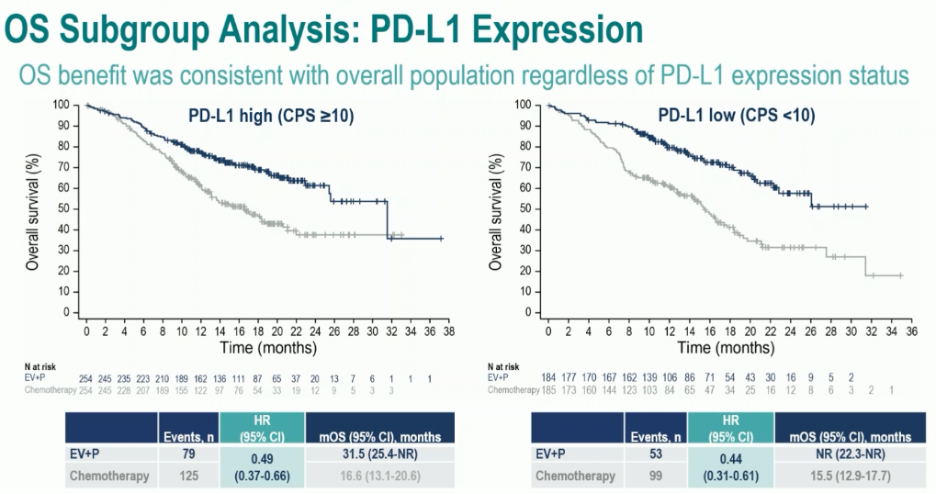
Significantly, clinically meaningful benefits for enfortumab vedotin + pembrolizumab were observed irrespective of cisplatin eligibility or tumoral PD-L1 expression:

A complete response was observed in 29% of patients in the enfortumab vedotin + pembrolizumab arm, compared to 12.5% for platinum-based chemotherapy. The objective response rates (ORRs) were 68% and 44%, respectively: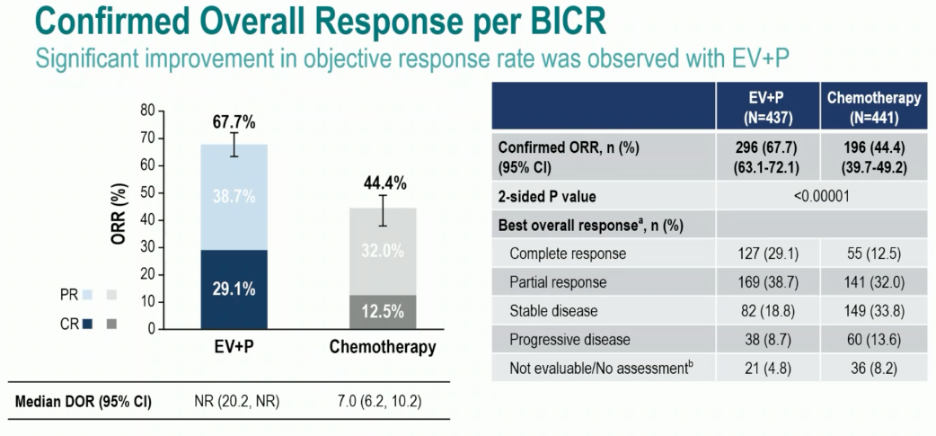
Treatment-related adverse events (TRAEs) were consistent with the known safety profiles of each of the drugs. Serious TRAEs occurred in 27.7% of patients in the enfortumab vedotin + pembrolizumab arm, compared to 19.6% with chemotherapy. TRAEs leading to death occurred in 4 patients (0.9%) in each arm. Overall, grade ≥3 adverse events occurred in 56% and 70% of patients in the enfortumab vedotin + pembrolizumab and chemotherapy arms, respectively. Notable TRAEs related to enfortumab vedotin were:
- Skin reactions (overall: 67%; Grade ≥3: 16%)
- Peripheral neuropathy (overall: 63%; Grade ≥3: 7%)
- Ocular disorders (overall: 21%; Grade ≥3: 0%)
- Hyperglycemia (overall: 13%; Grade ≥3: 6.1%)

Next, Dr. Apolo discussed the CheckMate 901 trial that randomized 608 cisplatin-eligible patients 1:1 to:
- Nivolumab + gemcitabine/cisplatin in 3-week cycles, up to a total of 6 cycles
- Nivolumab maintenance at 480 mg every 4 weeks was continued as maintenance therapy in responders until progression, unacceptable toxicity, withdrawal, or up to 24 months
- Gemcitabine + cisplatin at same doses/schedule/cycles
The trial design for CheckMate 901 is as follows: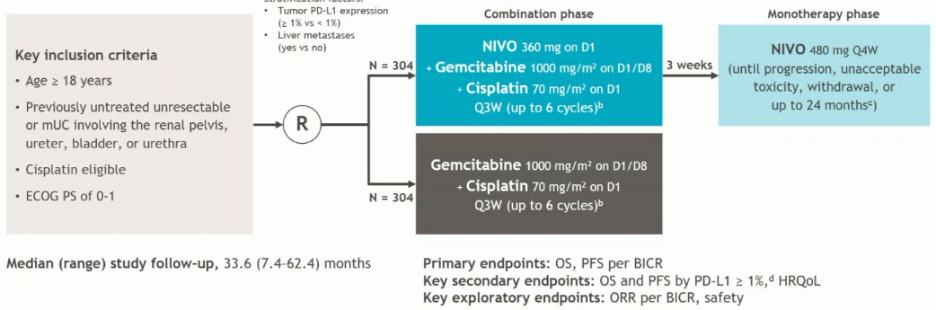
Similar to EV-302/KEYNOTE-A39, 25% of patients had upper tract disease. Overall, 37% of patients had a high tumor PD-L1 expression, defined as ≥1%, and 21% of patients had liver metastases.
The median study follow-up was 33.6 months, with 74% of patients in the nivolumab + gemcitabine/cisplatin arm completing all 6 cycles of therapy, compared to 55% of those in the control gemcitabine/cisplatin arm. In total, 80% of patients randomized to nivolumab + gemcitabine/cisplatin went on to receive maintenance nivolumab monotherapy.
The study met its primary endpoint of overall survival, with a median improvement of nearly 3 months (21.7 versus 18.9 months; HR: 0.78, 95% CI: 0.63 – 0.96, p=0.017):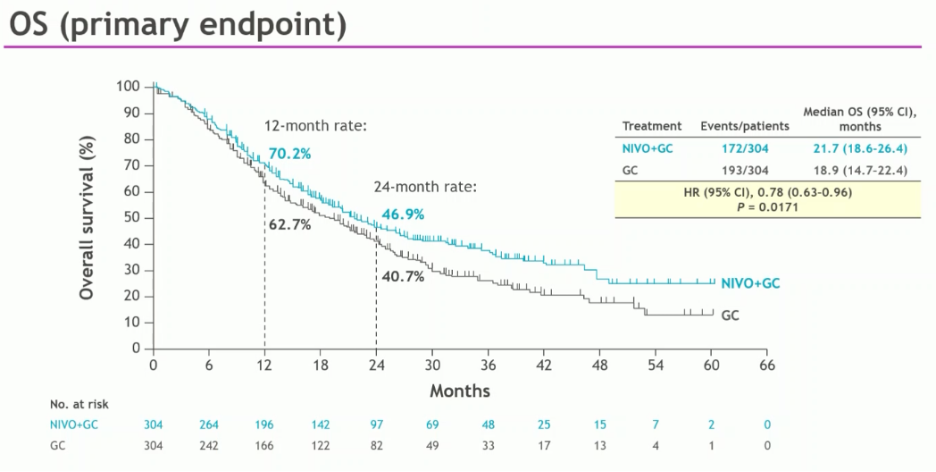
The ORR was higher with nivolumab +gemcitabine/cisplatin (58% versus 43%), with a higher proportion of patients achieving a complete response (22% versus 12%).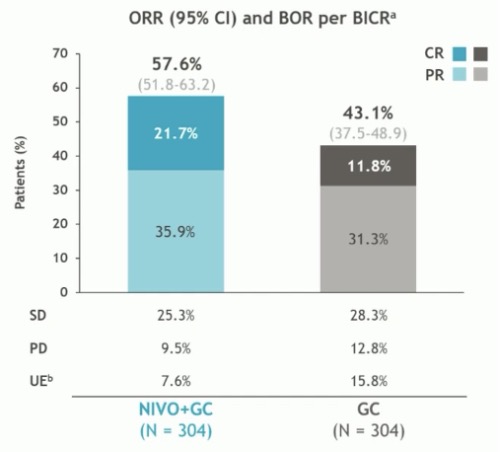
Contextualizing the results of these two trials, Dr. Apolo emphasized that the goals of treatment in the 1st line setting in metastatic urothelial carcinoma are:
- Early tumor shrinkage
- Durable responses
- Longer overall survival
- Minimal treatment toxicity
She noted that one potential criticism of the control arm in EV-302 is that almost half of patients received carboplatin due to cisplatin ineligibility. However, it appears that the ORR with carboplatin in 1st line phase 3 trials is comparable to that seen in cisplatin/gemcitabine or any platinum/gemcitabine treated patients at 41 – 42%, as summarized below: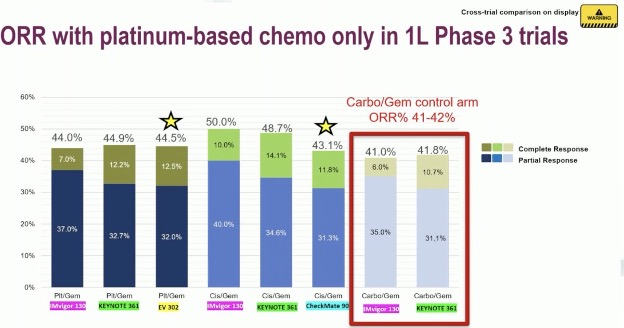
The combination of enfortumab vedotin + pembrolizumab has the highest ORR observed to date. The ORR was 68% with almost 30% of patients having a complete response. While cross-trial comparisons are fraught with limitations, these figures trump those observed in CheckMate-901 with nivolumab + gem/cis (58% and 22%, respectively) or other platinum-based chemotherapy combinations.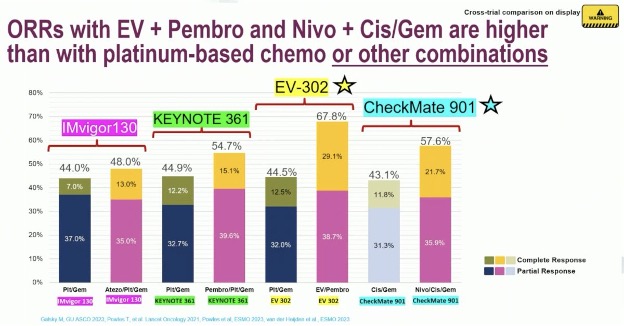
In addition to an improved ORR, the duration of response was also longest with enfortumab vedotin + pembrolizumab, with the median duration not yet reached with this combination, compared to a median of 9.5 months with nivolumab + gemcitabine/cisplatin.
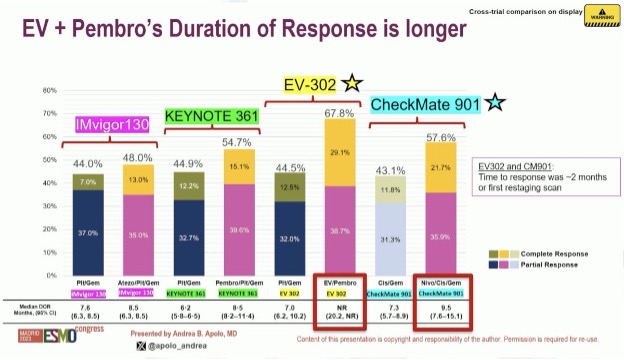
Most importantly, there are clear overall survival benefits with both nivolumab + gem/cis (CheckMate-901) and enfortumab vedotin + pembrolizumab (EV-302). CheckMate-901 is the 1st study of combination chemotherapy + immune checkpoint inhibitors to demonstrate a significant overall survival benefit in this setting (21.7 versus 18.9 months).
Why was CheckMate-901 positive and the other trials of chemotherapy + checkpoint inhibitors (IMVigor130 and KEYNOTE-361) negative? This may be related to the eligibility criteria, which included cisplatin ineligible patients in these two latter trials. A subgroup analysis of these trials clearly demonstrates an improvement when analysis is limited to those who received gemcitabine/cisplatin. Potential reasons for this preferential improvement with cisplatin, as opposed to carboplatin, maybe that cisplatin has been associated with immunomodulatory effects, potentially through the induction of immunogenic cell death. Correlative studies in IMvigor130 have shown that:
- Cisplatin, versus carboplatin, improved outcomes in patients with pre-treatment tumors exhibiting restrained adaptive immunity and
- Cisplatin induced transcriptional changes in circulating immune cells, including upregulation of antigen presentation and T-cell activation
As such, it appears that cisplatin does not induce, but actually ‘reboots’ a pre-existing immune response leading to a dramatic response in a small subset of patients.

It is important to note that both sequential (JAVELIN 100: avelumab maintenance)5 and combination chemotherapy + checkpoint inhibitors have efficacy in this disease space. We, however, cannot directly compare these studies with different patient populations, with JAVELIN-100 specifically including those patients responding to 1st line chemotherapy.
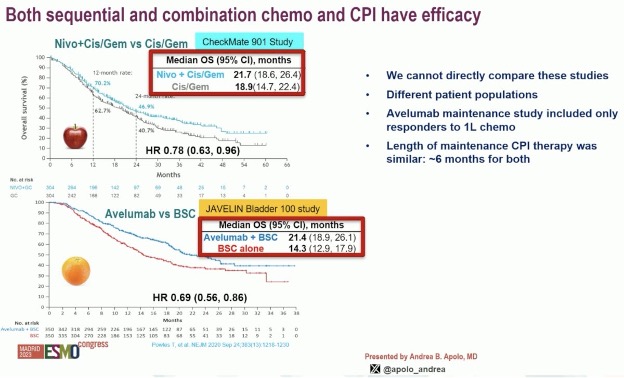
However, what is clear is that the combination of enfortumab vedotin + pembrolizumab far outperforms any other available combination for the 1st line treatment of unresectable or metastatic urothelial carcinoma. The median overall survival of 31.5 months in the experimental arm of EV-302 outperforms all other combinations.

Dr. Apolo noted that more patients in the control arm of EV-302 received maintenance checkpoint inhibition before progressive disease (32% versus 20% in CheckMate-901), and 59% received any subsequent checkpoint inhibitors (versus 40%). Given that approximately 30% of patients currently receive avelumab maintenance in clinical practice, these patterns are likely reflective of real-world practice.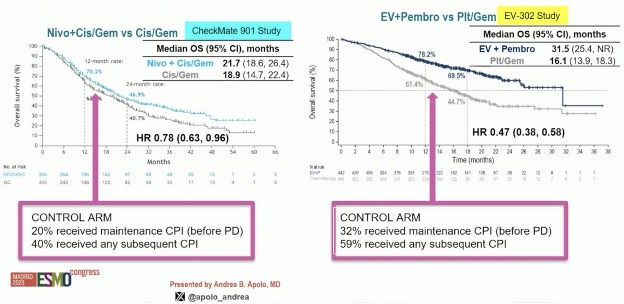
Based on these results, the FDA approved these two combinations as follows:
- December 2023: Enfortumab vedotin + pembrolizumab received full FDA approval for patients with locally advanced or metastatic urothelial cancer6
- March 2024: Nivolumab in combination with cisplatin and gemcitabine for first-line treatment of adult patients with unresectable or metastatic urothelial carcinoma7
These approvals have been reflected in the most recent update of the NCCN guidelines, whereby the combination of enfortumab vedotin plus pembrolizumab is now considered the preferred regimen for both cisplatin eligible and ineligible patients (category 1 for both).
This has also been reflected in the most recent updates of the EAU and ESMO guidelines.
Dr. Apolo concluded her presentation as follows:
- Two phase 3 trials in the 1st line treatment of advanced/metastatic urothelial carcinoma have demonstrated an improvement in overall survival:
- CheckMate 901 (nivolumab + gemcitabine/cisplatin) and EV-302 (enfortumab vedotin + pembrolizumab)
- The combination of nivolumab + gemcitabine/cisplatin is the first chemotherapy/immune checkpoint inhibitor combination to demonstrate a survival benefit with a median overall survival of 21.7 months.
- However, the combination of enfortumab vedotin + pembrolizumab almost doubled the median overall survival compared to chemotherapy (31.5 months versus 16.1), making it the new standard of care for patients in this setting.
- Treatment guidelines have recently been updated to include this major change in the treatment of metastatic urothelial carcinoma.
Presented by: Andrea B. Apolo, MD, Chief, Bladder Cancer Section Genitourinary Malignancies Branch Center for Cancer Research, National Cancer Institute, Bethesda, MD
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 BCAN Bladder Cancer Think Tank held in San Diego, CA between August 7th and 9th, 2024
References:- van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine–Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023;389:1778-89.
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024l390:875-88.
- Galsky MD, Arranz Arija JA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomized, placebo-controlled phase 3 trial. Lancet. 2020 May 16;395(10236):1547-57.
- Powles T, Csoszi T, Ozguroglu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomized, open-label, phase 3 trial. Lancet Oncol. 2021;S1470-2045(21)00152-2.
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383(13):1218-30.
- FDA approves enfortumab vedotin-ejfv with pembrolizumab for locally advanced or metastatic urothelial cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic-urothelial-cancer. Accessed on August 8, 2024
- FDA approves nivolumab in combination with cisplatin and gemcitabine for unresectable or metastatic urothelial carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-combination-cisplatin-and-gemcitabine-unresectable-or-metastatic-urothelial#:~:text=On%20March%206%2C%202024%2C%20the,metastatic%20urothelial%20carcinoma%20(UC). Accessed on August 8, 2024.


