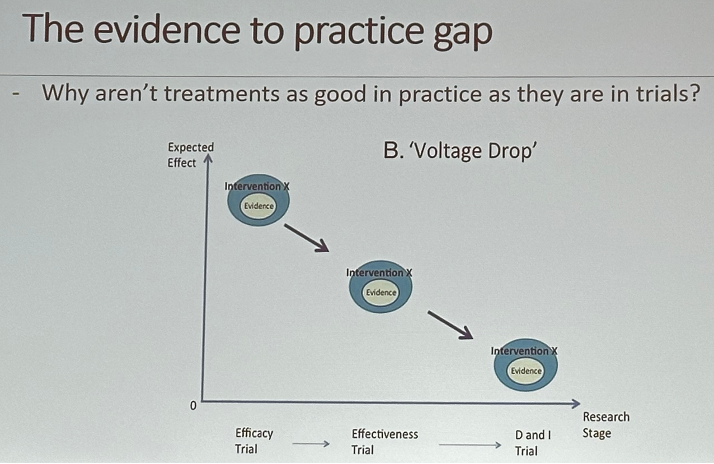
Why do we need implementation science? The effectiveness of treatments in the real world does not match the efficacy observed in clinical trials. Furthermore, it can take >17 years to incorporate advances into practice.
The goal of implementation science is to improve the delivery of evidence-based care by furthering our understanding of what strategies work best, when, for whom, and why. How do we get people to do the right things for the right patients at the right time? Additionally, when it does work – why does it work and how do we keep it going?
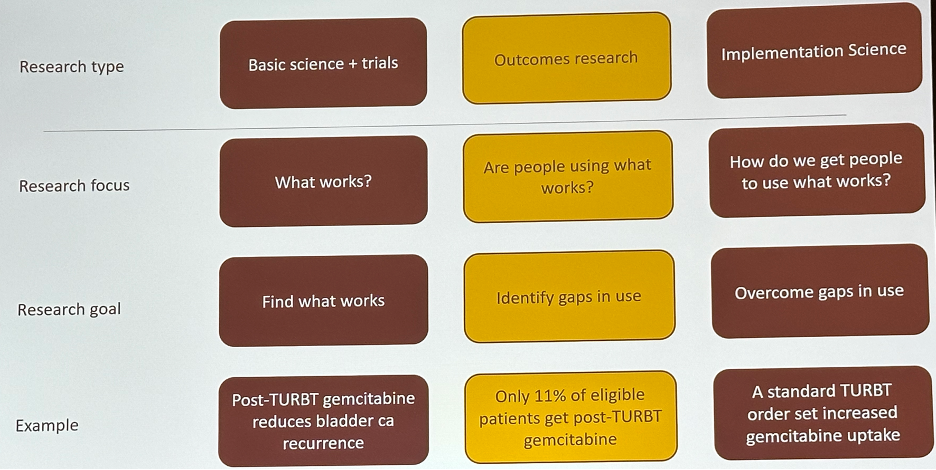
Dr. Stensland noted that we are great at coming up with new things (basic science and clinical trials) and describing how well we are doing the new things (outcomes research) but perform poorly with regard to working on improving how well we do new things (implementation science).

A simple framework to illustrate this ‘discord’ is the use of post-TURBT gemcitabine to reduce bladder cancer recurrence rates. Through clinical trials, it has been demonstrated that a single instillation of gemcitabine post-TURBT reduces bladder cancer recurrence rates.1 However, outcomes research studies have demonstrated that only 11% of eligible patients receive gemcitabine post-TURBT. How do we overcome gaps in its usage? One potential example of an implementation science technique would be to include post-TURBT gemcitabine as part of a standard TURBT order set.
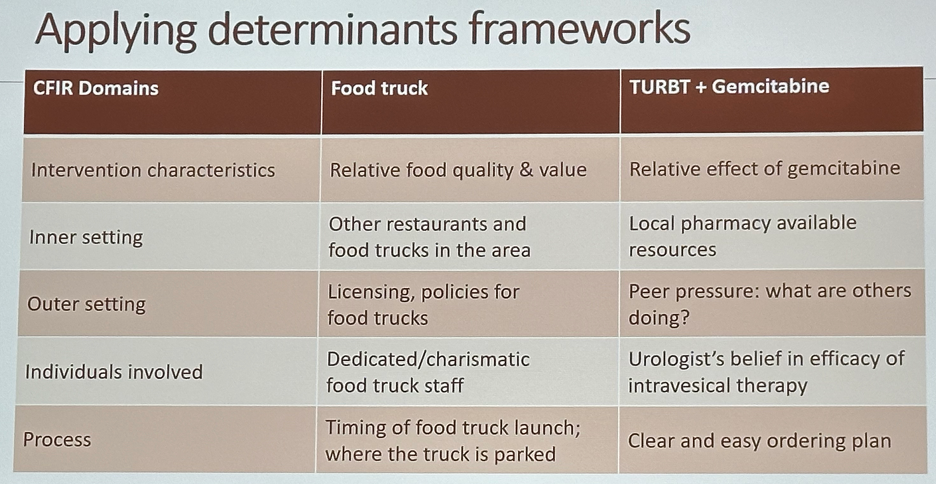
How do we approach such an implementation gap? Context assessment, at various levels, is critical to identify determinants, including both barriers and facilitators of successful implementation:
- Patient
- Provider/Group
- Organization
- Market/policy
- Consolidated Framework for Implementation Research (CFIR)
- Theoretical Domains Framework (TDF)
- Tailored Implementation in Chronic Disease (TICD)
- Intervention characteristics (e.g., relative effect of gemcitabine)
- Inner setting (e.g., local pharmacy available resources)
- Outer setting (e.g., peer pressure: what are others doing?)
- Individuals involved (e.g., urologist’s belief in the efficacy of intravesical therapy)
- Process (e.g., clear and easy ordering plan)
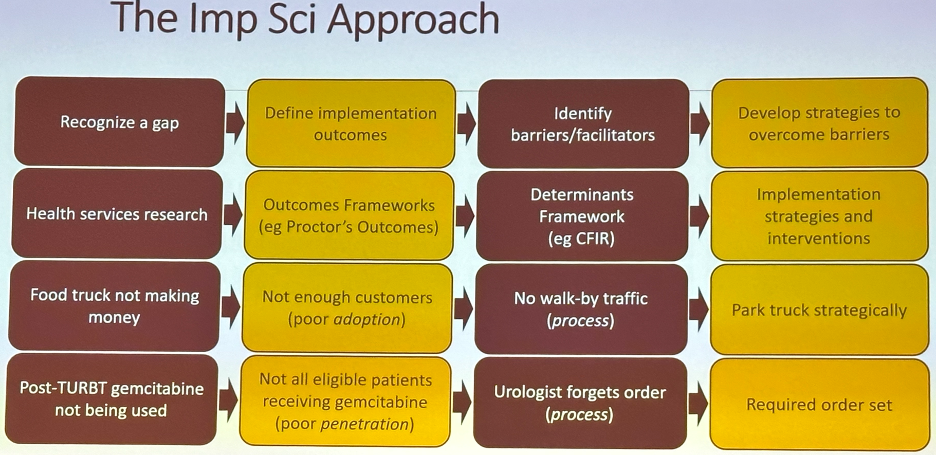
Why does identifying these determinants matter? This allows for targeted improvement strategies and saves time/resources for targeting specific barriers. For example, if gemcitabine is not available (domain: inner setting), then implementing pop-up reminders in the operating room or the pre-operative clinic will not help address this barrier to implementation.

A more efficient approach would be as follows:

After implementing changes based on the identified determinants, how do we identify if these implementation strategies are working? One common approach is to use implementation outcome frameworks, including:
- Proctor’s Outcomes
- RE-AIM
The next step after identifying the success of implementation strategies is to employ techniques that seek to maintain implementation outcomes by providing audit and feedback, developing incentives/disincentives, and reminding clinicians. There are nuances to this approach. For example, how do we deliver audit and feedback – by email, in a conference, or via a phone call? How frequently should it be delivered? This requires thoughtful consideration on a case-by-case scenario.
Dr. Stensland concluded that implementation science tries to improve the delivery of evidence-based care. It assesses context so that context-specific interventions can be designed using:
- Determinant frameworks
- Outcome frameworks
- Implementation strategies
Presented by: Kristian Stensland, MD, MPH, MS, Assistant Professor, Department of Urology, University of Michigan, Ann Arbor, MI
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 BCAN Bladder Cancer Think Tank held in San Diego, CA between August 7th and 9th, 2024
References:
- Messing E, Tangen CM, Lerner SP, et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. JAMA. 2018;319(18):1880-8.


