The NCCN is a not-for-profit alliance of 33 leading academic cancer centers in the United States devoted to patient care, research, and education. Its roles are as follows:
- Arbitrator of high-quality cancer care
- Develop and communicate scientific, evaluative information to better inform the decision-making process between patients and physicians
- Develop and promote national programs to facilitate the fulfillment of Member Institution missions
- Work to promote cancer care globally through policy, research, and education
The NCCN Guidelines are continuously updated and revised to reflect new data and clinical information. The ‘input’ for these guidelines and their updates relies on:
- Institutional review
- Clinical data review
- PubMed literature review
- Third-party submissions
- Scientific meetings/congress presentations
- Recent FDA approvals

Dr. Flaig next provided an explanation of the NCCN’s ‘recommendations’ and ‘preference’ nomenclatures.’ The NCCN categories of evidence and consensus are based on both the level of clinical evidence available and the degree of consensus within the NCCN Guidelines Panel, which considers evidence of efficacy and safety of interventions. All recommendations are ‘category 2A’ unless otherwise indicated.

‘Categories of preference’ provide guidance on which recommendations within the NCCN Guidelines are optimal within a range of recommendations to accommodate a variety of clinical circumstances. He emphasized that all recommendations in the NCCN Guidelines are considered appropriate.

It is important to note the NCCN Guidelines ‘Firewall’. The NCCN imposes strict policies to shield the guidelines development processes from external influences. The "firewall" surrounding the NCCN Guidelines processes includes:
- Financial support policies
- Panel participation and communication policies
- Guidelines disclosure policies
- Policies regarding relationships to NCCN's other business development activities
However, submission requests to NCCN guidelines panels are welcomed. External parties are invited to submit requests through the submission portal on NCCN's website for specific issues or topics to be discussed by the NCCN Guidelines Panel (NCCN.org/submissions). Examples of such parties include patient advocates, organizations, industry, and/or payers. External parties should always refer to this submission process and not contact Panel Members regarding any potential or suggested changes to the NCCN Guidelines.
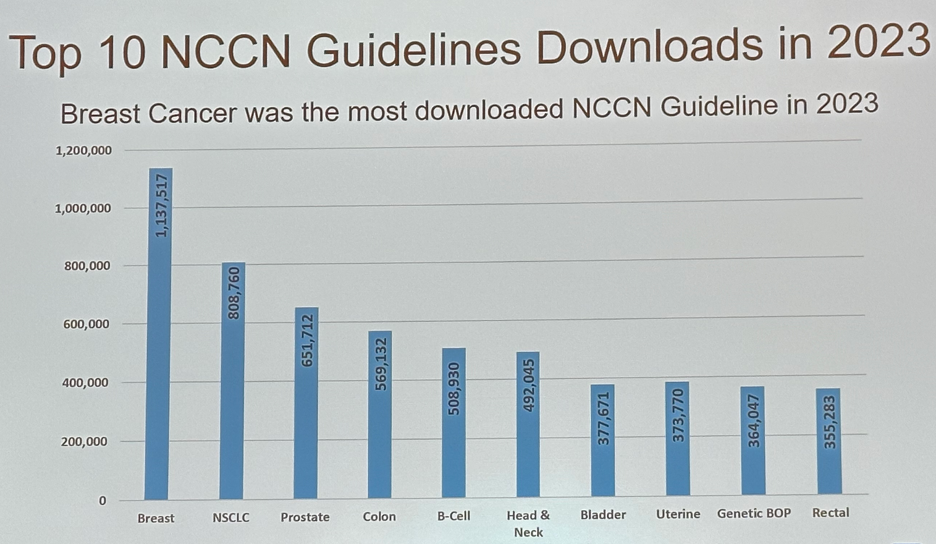
The impact of the NCCN Guidelines is reflected by the >15 million downloads registered in 2023.

The breast cancer guidelines were the most downloaded NCCN guidelines in 2023, with the bladder cancer guidelines ranking 7th overall.
He highlighted the evolution of the NCCN Bladder Cancer guidelines over the past decade, noting that in 2015 the state of advanced bladder cancer was limited to cisplatin combination chemotherapy for cisplatin-eligible patients, with carboplatin or taxane-based regimens considered as alternative regimens. Notably, there was no standard therapy in the 2nd line setting.

If we contrast this with the 2024 updated recommendations, we can see that the treatment paradigm for 1st line systemic therapy only ‘overwhelms’ all the treatment options that were available overall in 2015.

One unique aspect of the NCCN is its development and adoption of ‘Harmonized Guidelines’, which is an example of an international implementation approach. One of the goals of the NCCN Guidelines is to ensure awareness, accessibility, and relevancy of NCCN resources around the world. This included language translations of the guidelines, adoption of an NCCN FrameworkTM, NCCN Harmonized GuidelinesTM, and Regional Adaptations. The demand has grown for an NCCN guideline approach to cancer care in different resource settings and in specific geographic areas. This is reinforced by the fact that nearly half of the 1.8 million NCCN Guidelines registered users are from outside the United States.
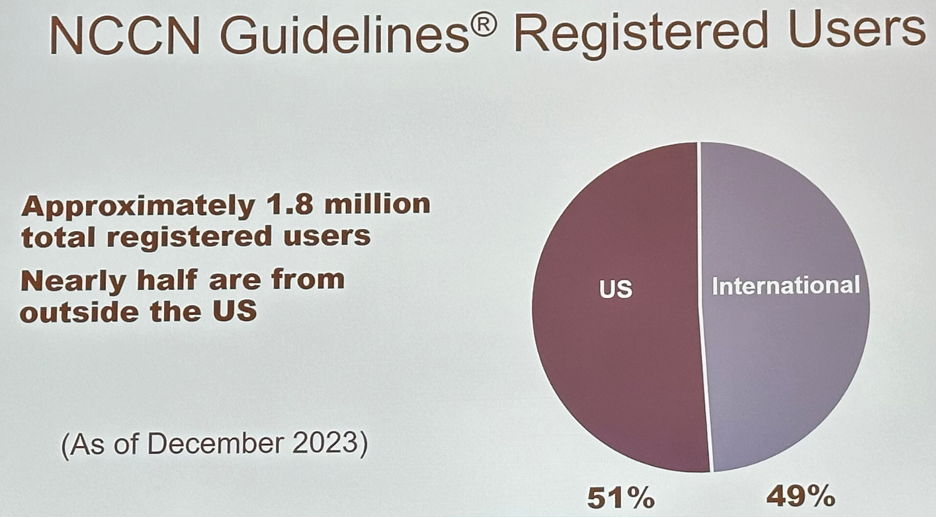
There are currently >800,000 international registered users, and >7 million international downloads in >190 countries.

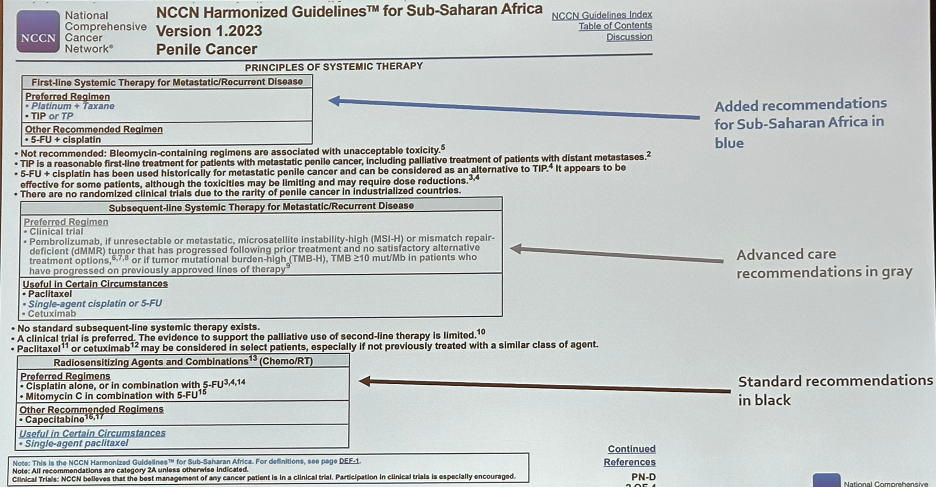
One example of the NCCN Harmonized Guidelines is that for Sub-Saharan Africa. These were created as a joint effort between the NCCN, African Cancer Coalition (ACC), American Cancer Society (ACS), and Clinton Health Access Initiative (CHAI). There are currently 54 published NCCN Harmonized Guidelines for Sub-Saharan Africa. The NCCN Harmonized Guidelines for Sub-Saharan Africa are now endorsed by the Federal Ministries of Health and leading cancer centers in Liberia, Ethiopia, Malawi, Nigeria, Tanzania, Uganda, and Zambia, representing 44% of the population of Sub-Saharan Africa
The NCCN Harmonized Guidelines are created to address unique regional needs and resources. They represent both the optimal care that low- and mid-resource countries aspire to provide and pragmatic approaches that provide effective treatment options for resource-constrained settings. The recommendations within the NCCN Harmonized Guidelines are represented as follows:

One example of these Harmonized Guidelines is that for penile cancer. Given that ifosfamide is often difficult to administer in these regions with many patients receiving cisplatin-paclitaxel (TP) only, these adapted guidelines highlight TP in italicized blue as a ‘regional option that may be considered when availability precludes general standard of care’.
In an effort to further improve treatment implementation, the NCCN has created the NCCN Guidelines for Patients that plainly explain these expert recommendations for people with cancer and their caregivers. There are currently >70 NCCN Guidelines for Patients, with over 1.4 million downloads in 2023.

Dr. Flaig concluded his presentation as follows:
- The NCCN supports a large clinical practice guidelines effort with broad uptake in the US and Internationally using a structured, well-supported development process
- The NCCN bladder cancer guidelines have accrued >350,000 downloads in 2023 and rely on a multi-disciplinary committee including urology, medical oncology, radiation oncology, pathology, and patient advocacy.
- There has been substantial effort to develop harmonized guidelines and other international outreach programs
- There has been a specific effort on patient guideline development
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 BCAN Bladder Cancer Think Tank held in San Diego, CA between August 7th and 9th, 2024


