(UroToday.com) The 2024 Bladder Cancer Advocacy Network (BCAN) Bladder Cancer Think Tank held in San Diego, CA was host to an interactive patient advocate-centered session addressing treatment options, concerns, and survivorship issues in a bladder cancer patient’s journey. After the BCAN patient advocates shared their bladder cancer journeys, Dr. Mary Beth Westerman provided an overview of contemporary surveillance regimens and tools for bladder cancer patients.
Current bladder cancer surveillance regimens rely on the combination of cystoscopy, cytology, and cross-sectional imaging. The rationale for surveillance is supported by the high recurrence rates for bladder cancer, known poor prognoses for early recurrences following BCG, and the potential ability to prevent progression when recurrences are identified early. Dr. Westerman noted that almost all current surveillance schedules and recommendations are based on expert opinion.
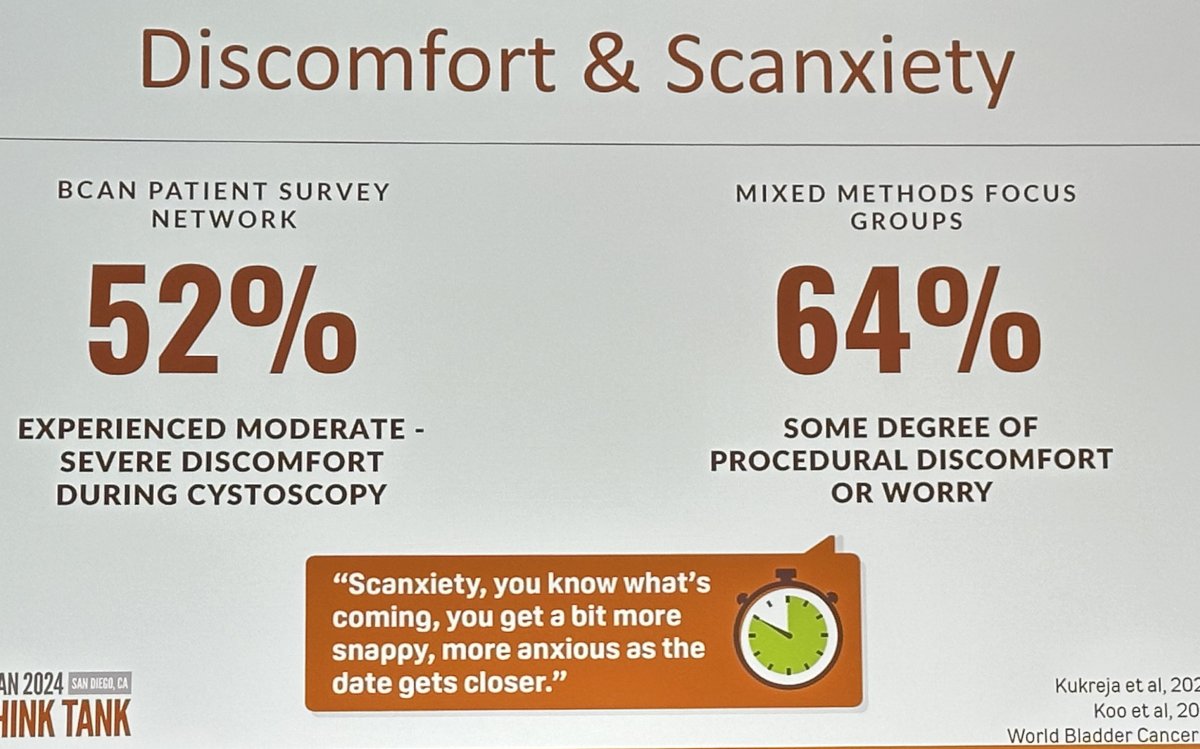
How do patients feel about current surveillance protocols? In a survey of the BCAN patient network, 52% reported experiencing moderate to severe discomfort during cystoscopy, and 64% of patients reported some degree of procedural discomfort or worry.1,2
We also need to consider the cost aspect, which includes copays and deductibles, missed work time, and transportation and parking costs.

As such, we need to balance the positives of early identification and progression prevention with potential discomfort, anxiety, increased cost, and lost time.

Currently, the AUA makes the following recommendations for risk-adapted surveillance of NMIBC patients:
- For a low-risk patient whose first surveillance cystoscopy is negative for tumor, a clinician should perform subsequent surveillance cystoscopy six to nine months later, and then annually thereafter; surveillance after five years in the absence of recurrence should be based on shared-decision making between the patient and clinician. (Moderate Recommendation; Evidence Strength: Grade C)
- For an intermediate-risk patient whose first surveillance cystoscopy is negative for tumor, a clinician should perform subsequent cystoscopy with cytology every 3-6 months for 2 years, then 6-12 months for years 3 and 4, and then annually thereafter. (Expert Opinion)
- For a high-risk patient whose first surveillance cystoscopy is negative for tumor, a clinician should perform subsequent cystoscopy with cytology every three to four months for two years, then six months for years three and four, and then annually thereafter. (Expert Opinion)
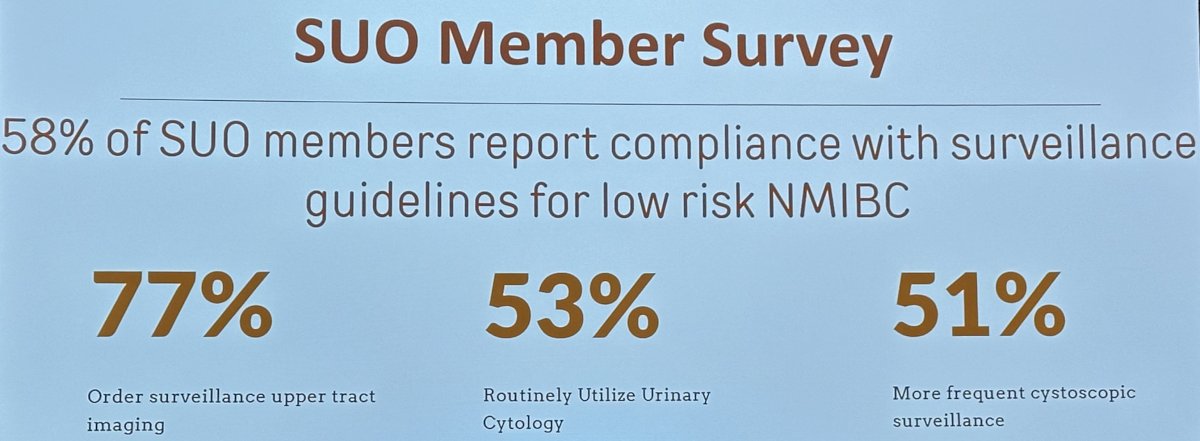
How well are physicians adopting/implementing these risk-adapted guidelines? In a 2020 published survey, only 58% of SUO members reported compliance with surveillance guidelines for low-risk NMIBC. 77% reported ordering surveillance upper tract imaging, 53% routinely utilized urinary cytology, and 51% performed more frequent cystoscopic surveillance than recommended by the guidelines.4
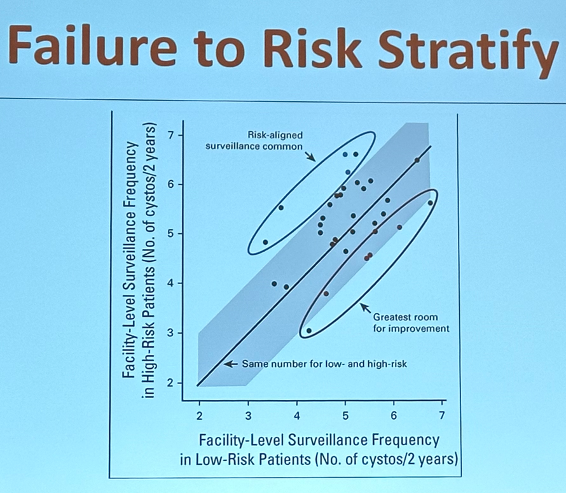
These findings are further supported by the important work carried out by Dr. Shroeck’s team that demonstrated a lack of risk-adapted surveillance regimen adoption for NMIBC patients in 81 of 85 evaluated Veterans Affairs (VA) medical centers.5
There has been an increase in the surveillance frequency of LG Ta lesions, with the annual cost of LG Ta NMIBC increasing by 60% between 2004 and 2013. This is due to an increase in the:
- Number of cystoscopies performed per year
- CT testing
- Cytology use
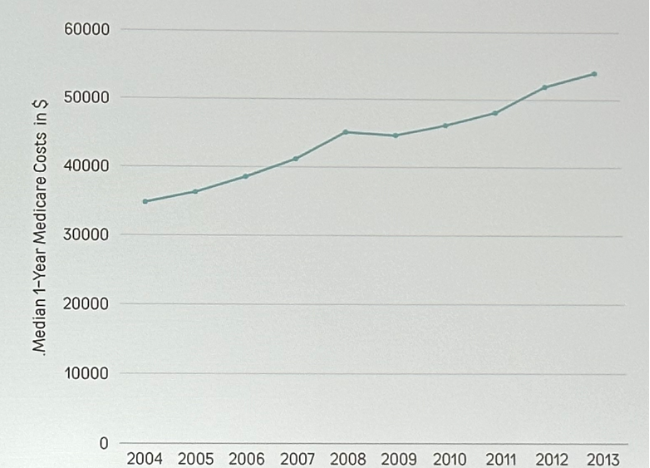
Notably, this has not coincided with an improvement in progression rates (0.4%).6
It appears that this ‘apprehension’ is not limited to physicians. In a 2021 randomized trial of low versus high-frequency surveillance, 35% of patients approached to participate in this trial declined participation due to their preference for high frequency surveillance scheduling.7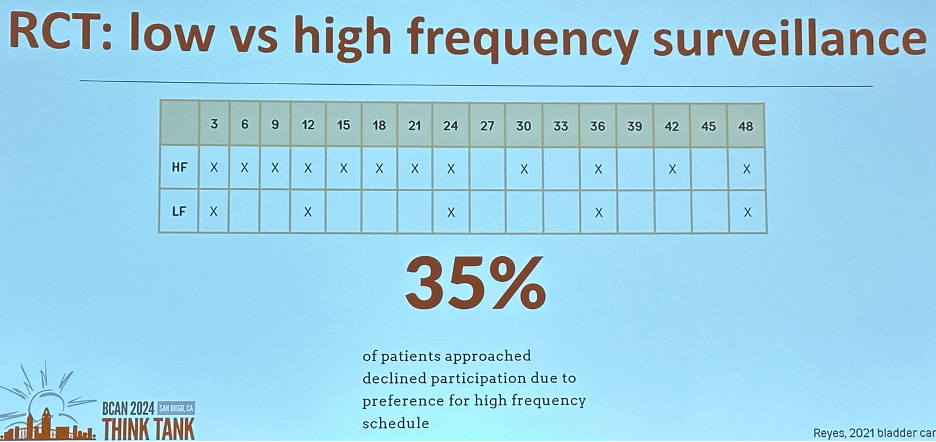
What about tumor markers? Ideally, we would have non-invasive urine-based tests available to identify patients who could safely defer surveillance cystoscopy. However, it appears that the patient-required benchmarks for these tests to replace cystoscopy are high. In a 2019 survey, 80% of patients demanded a minimum acceptable sensitivity of >99%, and 40% requested that it be 100%.8 A mixed methods study (DETECT II) demonstrated that 76% would accept a urine biomarker test with a sensitivity ≥90%, with patients prioritizing the tests with the highest sensitivity.9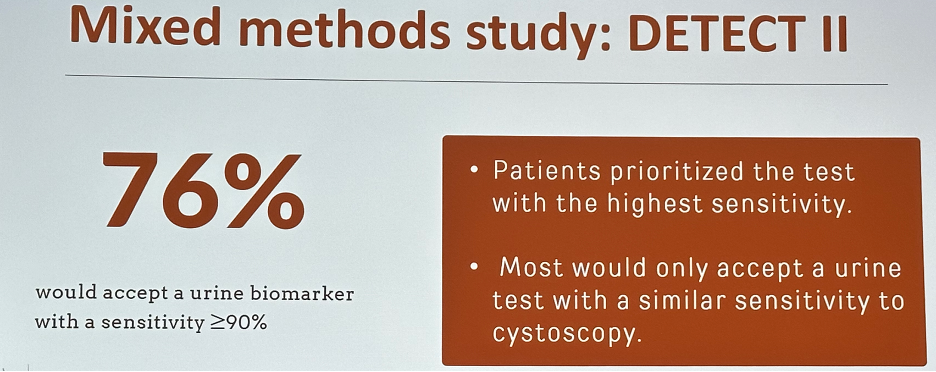
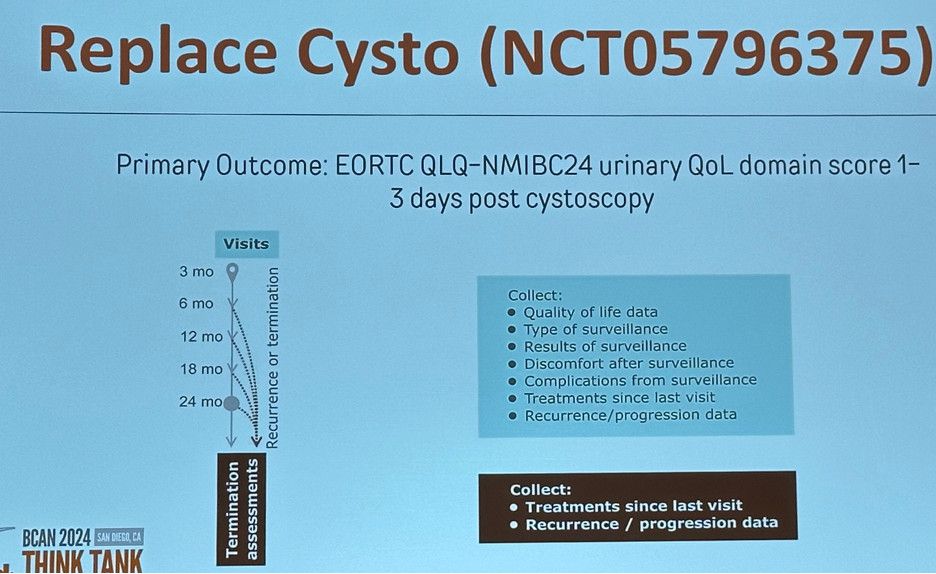
"Replace Cysto" is a multisite randomized phase 2 trial of 240 participants with low-grade intermediate-risk non-muscle-invasive bladder cancer, in which participants will be randomized 1:1:1 to one of two urine marker-based approaches alternating a urine marker test (Xpert Bladder Cancer Monitor or Bladder EpiCheck) with cystoscopy or to frequent scheduled cystoscopy.10 The primary objective is to determine whether urinary quality of life after surveillance is significantly improved in the urine marker arms. The primary outcome will be the patient-reported urinary quality of life domain score of the validated QLQ-NMIBC24 instrument, measured 1-3 days after surveillance. Exploratory outcomes will include:
- discomfort after surveillance
- Number of invasive procedures that participants undergo per 1000 person years
- Complications from these procedures per 1000 person years
- Non-urinary quality of life
- Acceptability of surveillance
- Bladder cancer recurrence and progression.
Presented by: Mary Beth Westerman, MD, Associate Professor, Department of Urology, LSU Health Sciences Center New Orleans, New Orleans, LA
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 BCAN Bladder Cancer Think Tank held in San Diego, CA between August 7th and 9th, 2024
References:
- Kukreja JB, Schroeck FR, Latan Y, et al. Discomfort and relieving factors among patients with bladder cancer undergoing office-based cystoscopy. Urol Oncol. 2022;40(1):9.e19-27.
- Koo et al, 2017, Urol World Bladder Cancer Patient Coalition.
- Holzbeierlein J, Bixler BR, Buckley DI, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline: 2024 amendment. J Urol. 2024;10.1097/JU.0000000000003846.
- Matulay JT, Tabayoyong W, Duplisea JJ, et al. Variability in adherence to guidelines based management of nonmuscle invasive bladder cancer among Society of Urologic Oncology (SUO) members. Urol Oncol. 2020;38(10):796.e6.
- Schroeck FR, Lynch KE, Chang JW, et al. Extent of Risk-Aligned Surveillance for Cancer Recurrence Among Patients With Early-Stage Bladder Cancer. JAMA Netw Open. 2018;1(5):e183442.
- Bree KK, Shan Y, Hensley PJ, et al. Management, Surveillance Patterns, and Costs Associated With Low-Grade Papillary Stage Ta Non–Muscle-Invasive Bladder Cancer Among Older Adults, 2004-2013. JAMA Netw Open. 2022;5(3):e223050.
- Reyes RM, Rios E, Barney S, et al. A Randomized Feasibility Trial Comparing Surveillance Regimens for Patients with Low and Low-Intermediate Risk Non-Muscle Invasive Bladder Cancer. Bladder Cancer. 2021;7(3):285-95.
- van Osch FHM, Nekeman D, Aaronson NK, et al. Patients choose certainty over burden in bladder cancer surveillance. World J Urol. 2019;37(12):2747-53.
- Tan WS, Sarpong R, Khetrapal P, et al. Exploring patients’ experience and perception of being diagnosed with bladder cancer: a mixed-methods approach. BJU Int. 2020;125(5):669-78.
- Schroeck FR, Grubb R, MacKenzie TA, et al. Clinical Trial Protocol for "Replace Cysto": Replacing Invasive Cystoscopy with Urine Testing for Non-muscle-invasive Bladder Cancer Surveillance-A Multicenter, Randomized, Phase 2 Healthcare Delivery Trial Comparing Quality of Life During Cancer Surveillance with Xpert Bladder Cancer Monitor or Bladder EpiCheck Urine Testing Versus Frequent Cystoscopy. Eur Urol Open Sci. 2024;63:19-30.


