(UroToday.com) The 2024 Bladder Cancer Advocacy Network (BCAN) Bladder Cancer Think Tank held in San Diego, CA was host to an interactive patient advocate-centered session addressing treatment options, concerns, and survivorship issues in a bladder cancer patient’s journey. This session was paneled by Drs. Roger Li and Mary Beth Westerman, and were joined by the following BCAN patient advocates:
- Joan Young
- Greg Kemp
- Doug MacLean
- Robert Serody
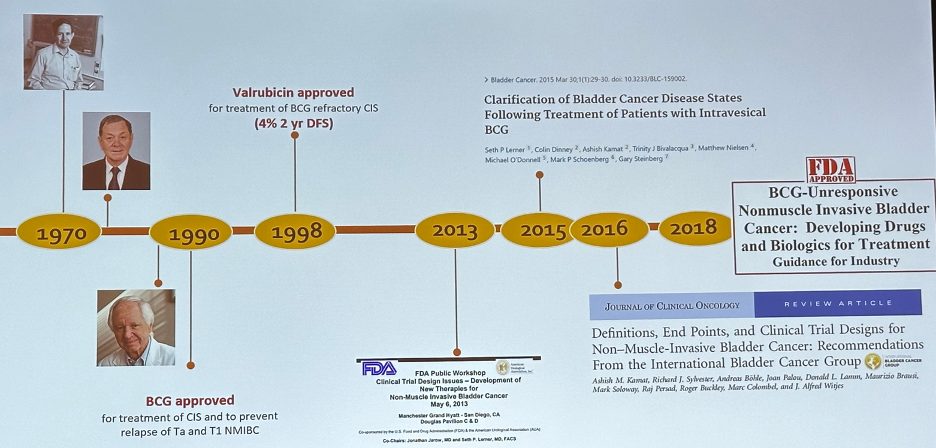
Prior to having the patient advocates share their bladder cancer journey, Dr. Asish Kamat provided a historic overview of the BCG unresponsive disease space. Studies of BCG therapy were underway in the 1970s, and BCG was approved for the treatment of CIS and prevention of Ta and T1 non-muscle invasive bladder cancer (NMIBC) relapse in 1990. In 1998, Valrubicin was approved for the treatment of BCG-refractory CIS. However, this drug had limited efficacy with a two-year disease-free survival rate of only 4%.
In 2013, the U.S. Food and Drug Administration (FDA) and the American Urological Association (AUA) co-sponsored a public workshop held in San Diego, CA to review potential trial designs for the development of new therapies for NMIBC.1 Prospective benchmarks for potential agent approvals in this space were set as follows:
- 6 months complete response rate: 40–50%
- 18–24 months durable response: ≥30%, with the 95% confidence interval excluding 20%

Dr. Kamat noted that this was considered to be ‘too optimistic’ by many experts in the field and that long-term disease-free rates of even 10% would be acceptable, particularly given the scarcity of available options at that time.
In 2015, the FDA rejected the application for approval of intravesical MCNA, which demonstrated disease-free survival rates of 25% and 19% at one and two years, respectively.2
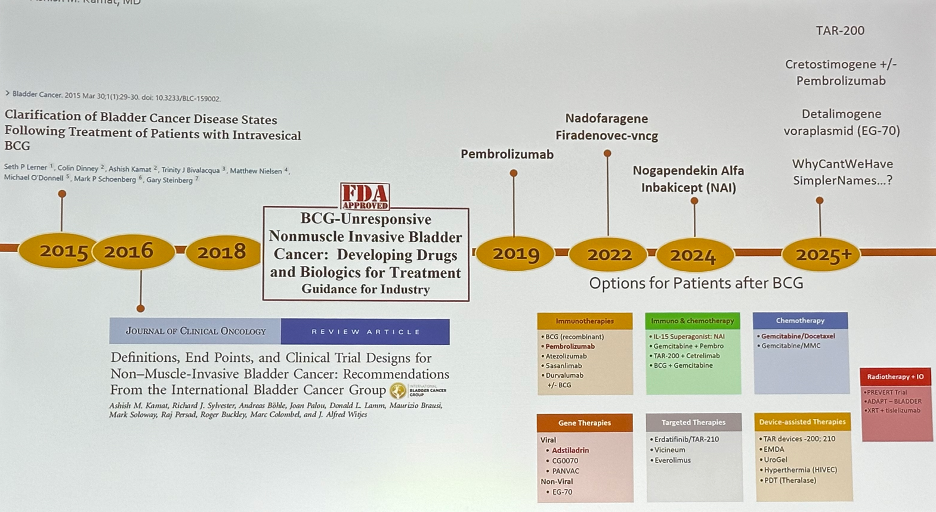
In 2016, the International Bladder Cancer Group (IBCG) published a landmark paper in the Journal of Clinical Oncology that provided guidance on definitions, endpoints, and clinical trial designs for NMIBC.3 This was followed in 2018 by the FDA publishing its guidance report for the industry for developing drug and biological products for the treatment of BCG-unresponsive NMIBC.
In 2019, pembrolizumab was approved by the FDA for BCG-unresponsive NMIBC. This was followed by the approval of Nadofaragene Firadenovec-vncg and Nogapendekin Alfa Inbakicept (NAI) in 2022 and 2024, respectively.
What matters most to patients? Data presented at GU ASCO 2021 highlighted that, in ascending order, patients care most about a complete response, 2-year recurrence-free survival, and preserving their bladder. Their tolerance for toxicity depends on whether events were reversible or permanent – 68% for reversible adverse events and 15% for permanent ones.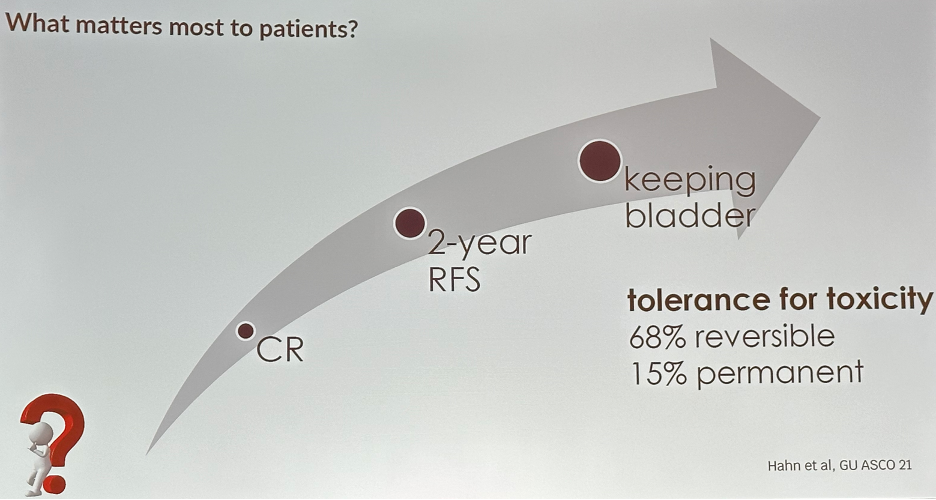
How can we frame the efficacy outcomes of currently available agents in the BCG-unresponsive NMIBC space with reference to the historical benchmarks set by the IBCG? The figure below visually illustrates the performance of these agents, in reference to these benchmarks. Gemcitabine + docetaxel (gem/doce) clearly exceeds these benchmarks. However, we note that the efficacy outcomes of gem/doce are based on the results of retrospective studies only. The other agents to have met or exceeded these benchmarks are nadofaragene and N-803 + BCG.4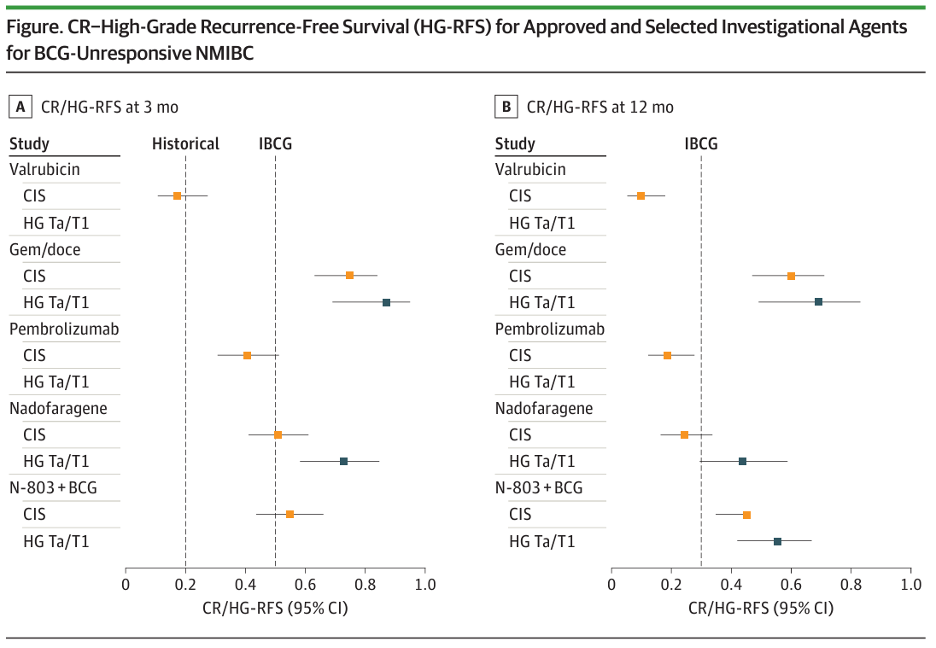
However, when we consider recently published data from MD Anderson experience that demonstrates that a repeat BCG challenge in patients who had failed prior BCG treatment is associated with 3- and 12-month recurrence-free survival rates of 75% and 69%,5 this raises the question of whether we need to ‘adjust’ our benchmarks for novel agents in this space.
To conclude, Dr. Kamat noted that many questions remain pertinent in this field, as summarized in the schematic below, and turned the stage over to the BCAN patient advocates to share their relevant experiences throughout their bladder cancer journeys.
Presented by: Ashish M. Kamat, MD, MBBS, Endowed Professor of Urologic Oncology (Surgery) and Cancer Research, Department of Urology, University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 BCAN Bladder Cancer Think Tank held in San Diego, CA between August 7th and 9th, 2024
References:
- Jarow JP, Lerner SP, Kluetz PG, et al. Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: report of a Food and Drug Administration and American Urological Association public workshop. Urology. 2014;83(2):262–4.
- Morales A, Herr H, Steinberg G, et al. Efficacy and safety of MCNA in patients with nonmuscle invasive bladder cancer at high risk for recurrence and progression after failed treatment with bacillus Calmette-Guérin. J Urol. 2015;193(4):1135-43.
- Kamat AM, Sylvester RJ, Bohle A, et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol. 2016;34(16):1935-44.
- Hwang TJ, Davies BJ, Preston MA. Advancing Patient-Centered Outcomes and Equity in Clinical Trials for BCG-Unresponsive Nonmuscle Invasive Bladder Cancer. JAMA Oncol. 2023;9(11):1491-2.
- Myers AA, Tan WS, Grajales V, et al. Challenging the Paradigm of "BCG-unresponsive" Bladder Cancer: Does Additional Bacillus Calmette-Guérin Have an Effect? Eur Urol. 2024:S0302-2838(24)02409-6.


