(UroToday.com) The 2024 Bladder Cancer Advocacy Network (BCAN) Bladder Cancer Think Tank held in San Diego, CA was host to a 2022 and 2021 Bladder Cancer Research Innovation Awardees session. Dr. Tyler Curiel presented the results of an analysis evaluating rationally engineered IL-2 for the enhanced treatment of local and metastatic bladder cancer.
The IL-2 receptor is composed of three components: the α (IL-2Rα), β (IL-2Rβ), and common γ (γc) chains. To achieve high-affinity binding to IL-2, all three chains are required. It binds IL-2 with high affinity on activated T cells and regulatory T cells. The intermediate affinity receptor is made up of the IL-2β and γc chains. It binds IL-2 with intermediate affinity, mainly on memory T cells and NK cells. Engineered IL-2 would ideally bind to the intermediate affinity receptor leading to the activation of effector T-cells and block binding to the high affinity receptor inhibiting activation of regulatory T cells.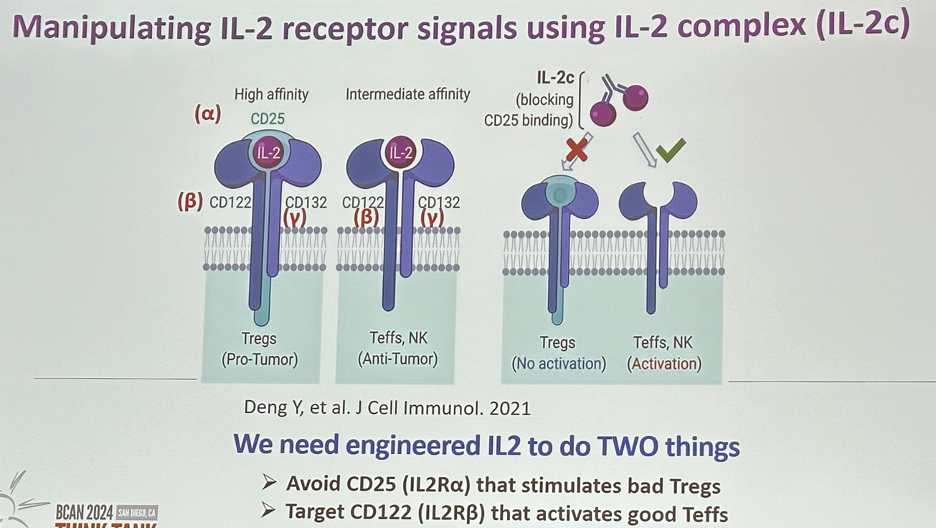
IL-2c is very effective against orthotopic bladder cancer and treats lung metastases in combination with an anti-PD-L1 agent. Importantly, IL2 activates adaptive immunity to treat bladder cancer.1
There are important limitations to high-dose IL-2:
- Stimulatory effects on regulatory T-cells
- Short half-life
- Vascular leak syndrome
Clinical challenges include:
- Dosing ratio optimization
- Complex stability concerns
Engineered IL-2 constructs and approaches have focused on avoiding CD25 and hitting CD122 instead. Low-dose IL-2 and improved IL-2 formulations with CD25 bias preferentially stimulate the trimeric IL-2 receptor consisting of CD25 and, thus, expand regulatory T-cells. On the other hand, high doses of IL-2 or CD122-biased IL-2 formulations preferentially stimulate the dimeric IL-2 receptor consisting of CD122 and CD132 and expressed on effector-type lymphocytes, such as resting antigen-experienced (memory) T and natural killer cells. Stimulation of these effector immune cells improves anti-tumour responses in cancer patients. Another approach focuses on the delivery of IL-2 to either the tumour microenvironment or anti-tumour effector immune cells by using targeted IL-2 formulations.2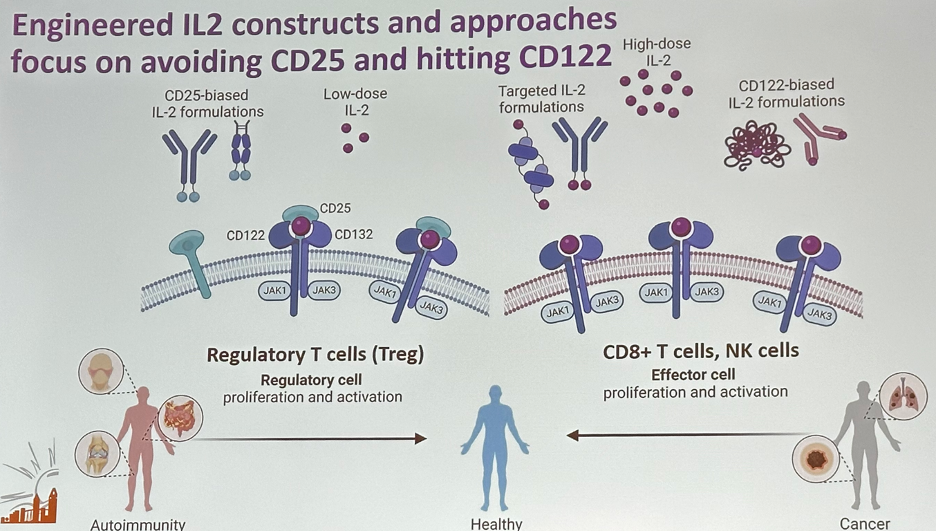
Most groups have tested IL-2 to improve effector T cells, but immune memory is also required for durably effective immunotherapy. It has been shown that IL-2 receptor signal strength also dictates effector versus memory cell differentiation.3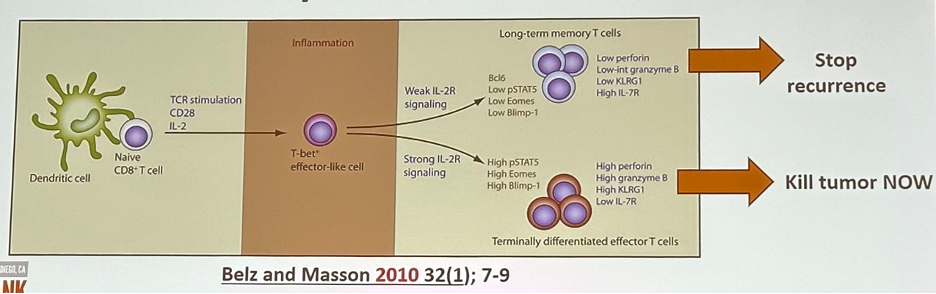
Ideally, we would develop IL-2 constructs with optimal memory and effector potency. The engineered IL-2 would do three things:
- Avoid CD25 (IL2Rα) that stimulates bad Tregs
- Target CD122 (IL2Rß) that activates good Teffs
- Jigger IL-2 receptor signals for the right balance of T effector and memory cells
- BONUS: greatly activate innate anti-bladder cancer immunity
The engineered H9 (mutated IL-2) was shown to have 220-fold reduced binding to CD25 (anti-CD25 biased). It is engineered for lower IL-2 receptor signals favoring memory T-cell bias.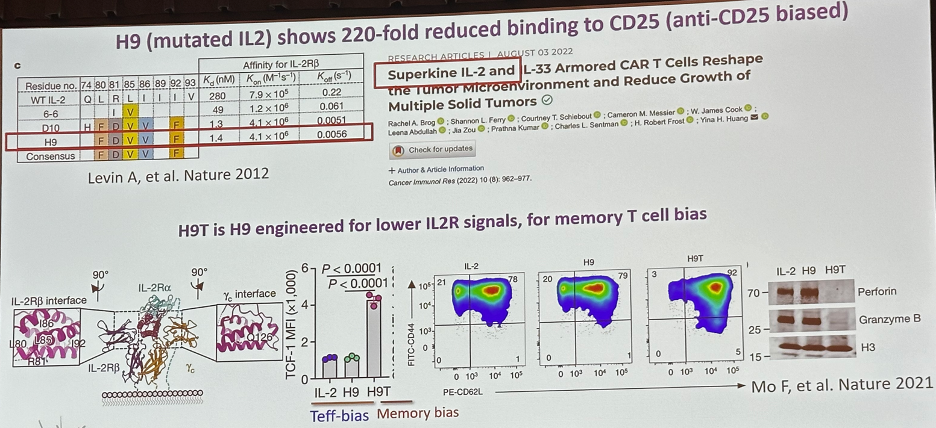
Dr. Curiel’s team engineered mutated IL-2s to fuse to antibodies to achieve all three aforementioned objectives: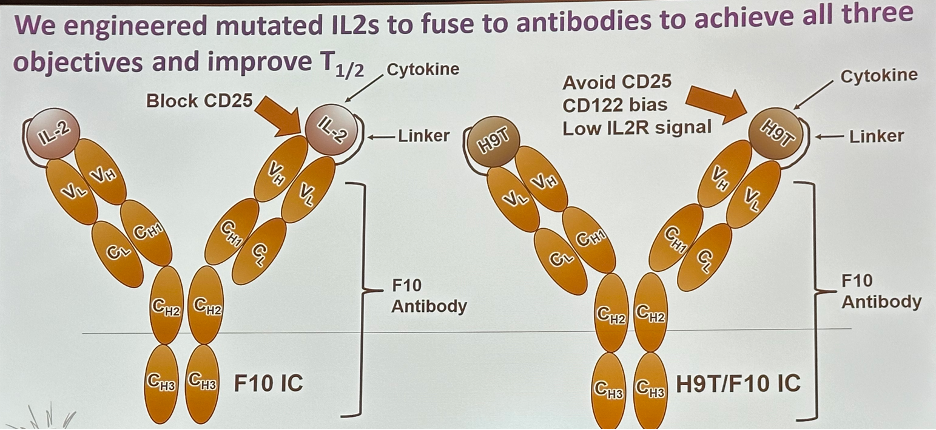
These engineered constructs demonstrated the desired IL-2 receptor binding and signal effects.
Do engineered IL-2 molecules elicit memory T cells in vitro? Dr. Curiel’s team demonstrated that engineered H9T induces stem cell and central memory T cells in vitro.
The next question is: do these engineered IL-2 molecules exhibit therapeutic efficacy in vivo? They were able to demonstrate in mouse models that engineered IL-2 effectively treats subcutaneous bladder cancer, with minimal side effects (e.g., extravasation).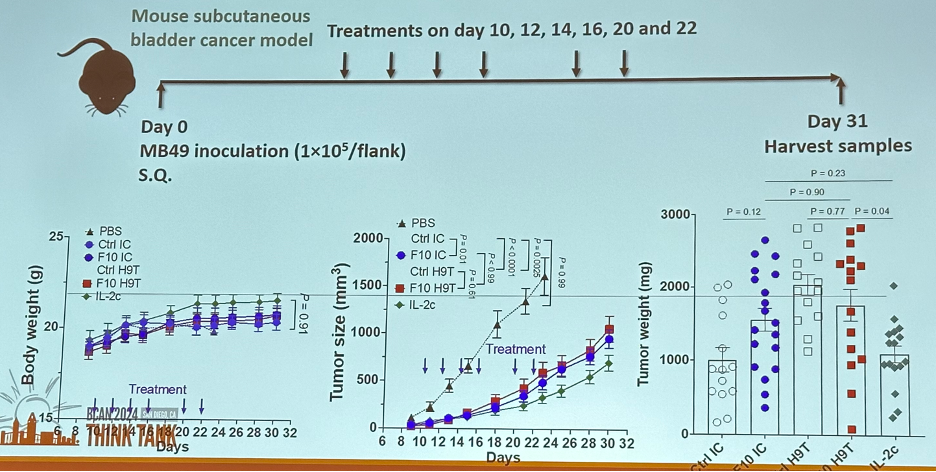
Furthermore, memory-biased engineered IL-2 compounds induce T resident memory in vivo.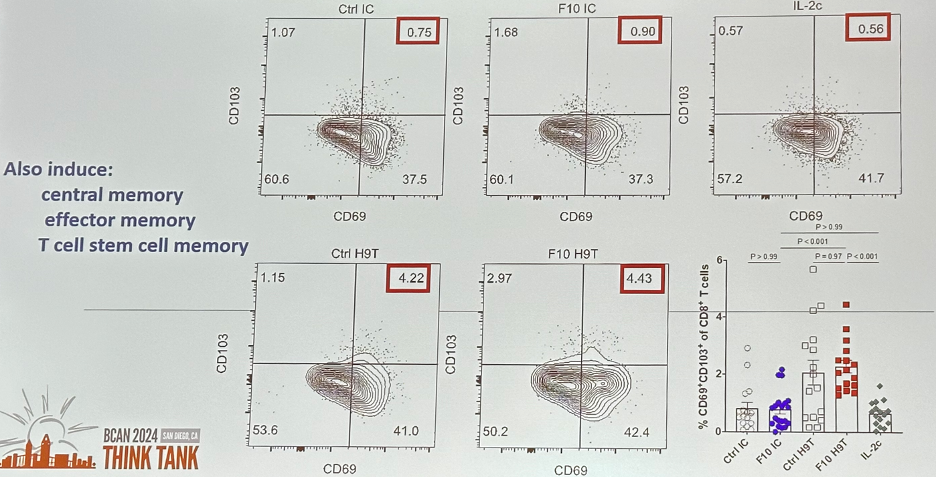
Ideally, we would be able to engineer one IL-2 construct that has both optimal effector T-cell and memory T-cell stimulatory effects. However, until that becomes available, one approach is to combine IL-2 molecules that individually generate effector-biased and memory-biased IL-2 molecules. Can we give effector-biased plus memory-biased IL-2 molecules to exploit the strength of each in vivo? Will the order of treatment matter?
That is – memory-biased first versus effector-biased first?
In a mouse model illustrated below, Dr. Curiel’s team was able to demonstrate that administering H9T first induces more T cell memory. However, administering 3x IL-2/F10 (effector T-cell biased) followed by 3x H9T/F10 (memory T-cell biased) induces more cytotoxic
T-lymphocytes activates the innate immunity better.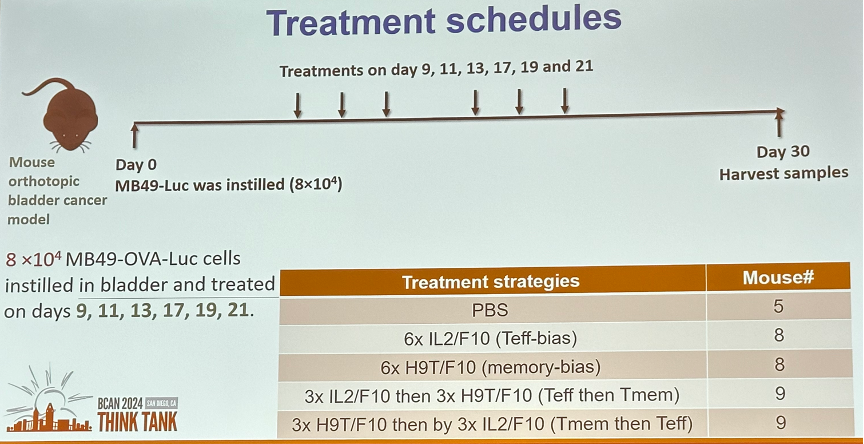
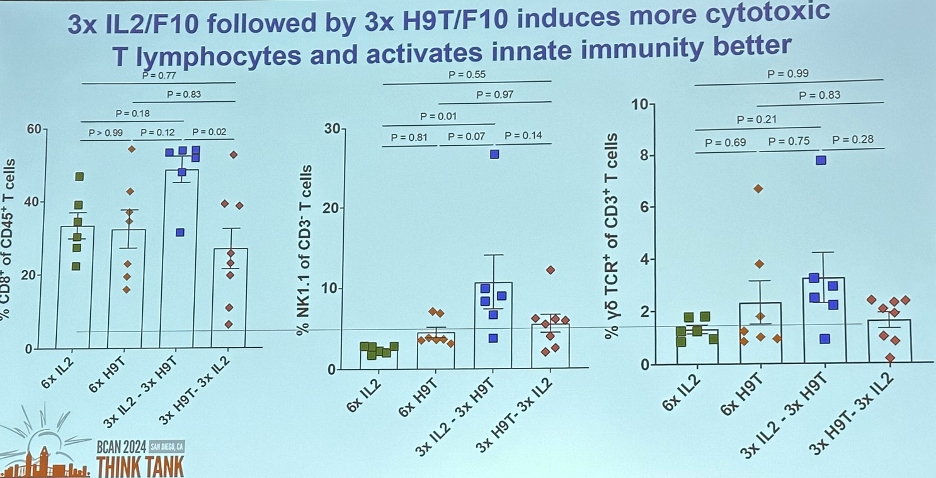
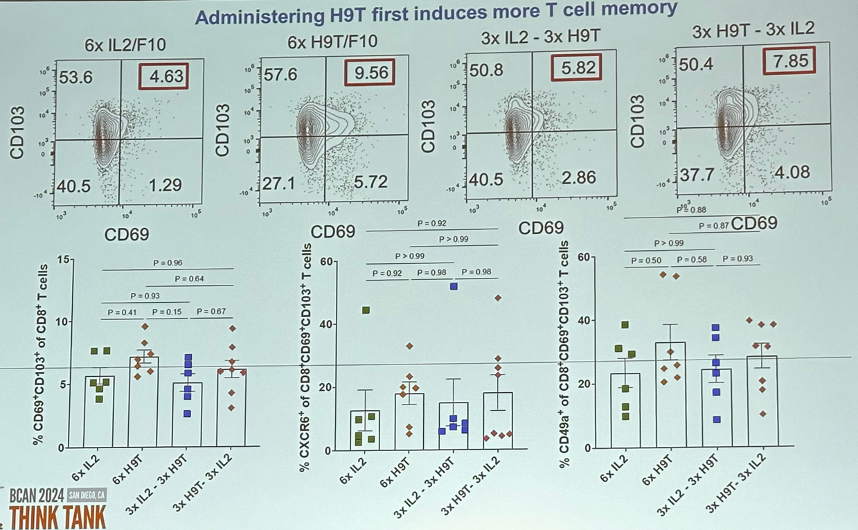
Dr. Curiel concluded that:
- All antibody-engineered IL-2 molecules control orthotopic bladder cancer.
- Engineered IL-2 in engineered antibody molecules retains functional characteristics of Tmemory bias.
- H9T engineered to antibodies elicits T resident memory and this effect in tumor may be better than other IL-2 molecules.
- Teff-bias followed by Tmem-bias treatment has potential to combine better with immune checkpoint blockade (e.g., anti-PD1) based on memory induction.
Presented by: Tyler J. Curiel, MD, MPH, Professor of Medicine, Department of Medicine, Dartmouth-Hitchcock Clinic, Hanover, NH
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 BCAN Bladder Cancer Think Tank held in San Diego, CA between August 7th and 9th, 2024
References:
- Reyes RM, Deng Y, Zhang D, et al. CD122-directed interleukin-2 treatment mechanisms in bladder cancer differ from αPD-L1 and include tissue-selective γδ T cell activation. J Immunother Cancer. 2021;9(4):e002051.
- Raeber ME, Sahin D, Karakus U, Boyman O. A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases. eBioMedicine. 2023;90:104539.
- Belz GT, Masson F. Interleukin-2 tickles T cell memory. Immunity. 2010;32(1):7–9.


