The purpose of these new guidelines is to provide a risk stratified clinical framework for the management of NMIBC. This risk stratification approach aids personalized treatment decisions and surveillance strategies as opposed to a generalized “one size fits all approach”.
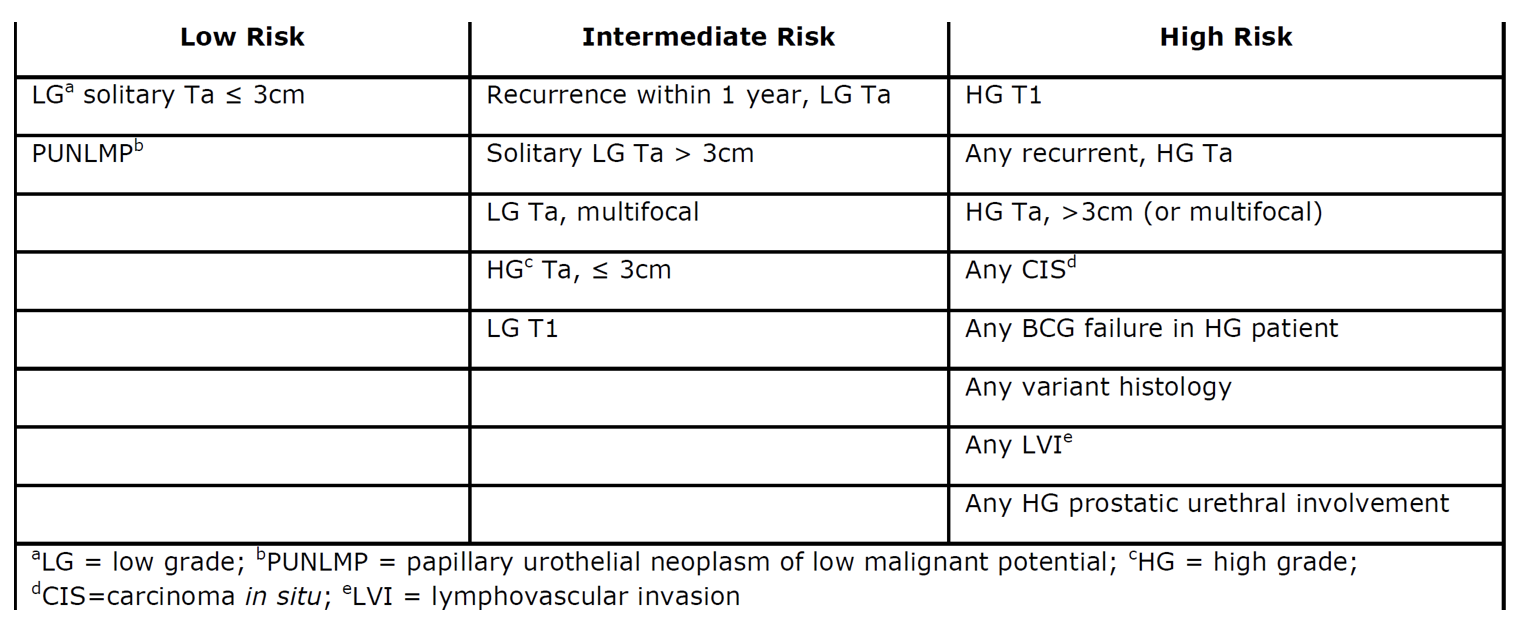
The risk stratification table of NMIBC is seen below. The first step in TURBT should be to assess and document the tumor size, location, configuration, number and mucosal abnormalities. A complete resection of all visible tumors must be done to lower risk of recurrence. Dr. Chang stressed the importance of evaluating the urethra as well, especially when CIS and/or invasive disease is present. He also emphasized usage of all available technologies in a judicious way, the performance of restaging TURBT within 6 weeks for HG disease, (for Ta and T1), and lastly, the importance of experience (younger surgeons are more likely to leave residual tumor lack muscle in the specimen).
Dr. Chang also stressed the importance of prostatic urethra biopsies at the 5 and 7 o’clock area, adjacent to the verumonatnum, in order to detect CIS. This is the area with the highest concentration of prostatic ducts, where CIS most likely is found. TUR of this area should provide a full thickness evaluation to rule out invasion.
It is recommended to use high definition imaging, clearly enabling visualization of more lesion and with more detail. Regarding using monopolar or bipolar electrocautery, randomized trials have shown no difference in results aside from better pathology specimens in the bipolar modality with much leas cautery artifacts. Blue light cystoscopy or narrow band imaging should be offered at the time of TURBT, to increase detection and reduce recurrence.
Imaging of the upper tract should be performed in bladder cancer patients. In patients with positive cytology and normal cystoscopy with a history of NMIBC, prostatic urethral biopsies should be performed as well as upper tract imaging, and enhanced cystoscopic modalities (such as blue light cystoscopy).
When encountering variant histology, an experienced genitourinary pathologist should review the pathology. Additionally, if bladder sparing approach is planned for a patient with variant histology, a restaging TURBT should be planned within 6 weeks of the initial TURBT. However, due to the high rate of upstaging associated with variant histology in bladder cancer, clinicians should consider offering initial radical cystectomy (RC) to these patients.
The AUA guidelines for NMIBC clearly state that usage of urinary biomarkers should not be done in place of cystoscopy evaluation (grade B recommendation). Furthermore, in patients with low risk cancer and a normal cystoscopy, urine cytology or a urinary biomarker should not be used during surveillance. However, in a NMIBC patient who was treated with BCG, biomarkers may be used to assess response (UroVysion FISH).
Restaging TURBT (with muscularis propria included in the specimen) should be done within 6 weeks of the initial TURBT in incomplete resection, in T1 HG disease (Grade B), and strongly considered in Ta HG disease (Grade C).
A single postoperative Intravesical instillation of chemotherapy (Mitomycin or Epirubicin) should be given to patients with known low or intermediate risk bladder cancer, within 24 hours of TURBT. Contraindications include suspected perforation and extensive resection. In low risk disease, induction intravesical therapy should not be administered, but in intermediate risk patients, a 6 week course of induction intravesical chemotherapy or immunotherapy should be considered. In high risk patients a 6 week induction course of BCG should be given.
No evidence to date exists to recommend one BCG strain over the other, or to recommend BCG in addition to another intravesical agent. In intermediate and high risk patients who respond to induction BCG therapy, maintenance therapy should be considered for one and three years, respectively. If an intermediate/high risk patient has persistent/recurrent disease or positive cytology following BCG therapy, prostatic urethral biopsies and upper tract imaging should be performed prior another induction course. A 2nd BCG induction course should be given to patients with persistent/recurrent Ta HG or CIS disease. However if T1 HG disease recurs after single BCG induction therapy, RC should be offered. BCG unresponsive is defined by the FDA as recurrent/persistent disease after completion of at least induction and one cycle of maintenance (5+2) for Ta/T1 HG or CIS disease. The patient should never have reached complete response or has recurred within 6 months of last BCG, or had a T1 HG disease at first evaluation after induction BCG.
RC for low/intermediate risk disease should not be offered until bladder sparing modalities have failed. In contrast, in high risk patients fit for surgery with persistent T1 HG disease, or associated CIS/LVI/variant histologies, initial RC should be offered. Lastly, in high risk patients with persistent/recurrent disease within one year following 2 induction cycles or BCG maintenance, RC should be offered.
Dr. Chang concluded his informative presentation with a summary on the AUA guidelines recommendations on NMIBC follow-up. The initial step in the follow-up of NMIBC includes first cystoscopy at 3-4 months after TURBT. Routine upper tract surveillance imaging in low risk asymptomatic patients should not be done, while in intermediate/high risk patients, it should be done every 1-2 years. Patients with a history of LG Ta disease and a noted sub-centimeter papillary tumor should undergo in-office fulguration. In low risk patients with a first normal cystoscopy after TURBT, the next cystoscopy should be within 6-9 months and then annually thereafter. Upon completion of 5 years of normal follow-up, further surveillance should be decided based on a shared decision making between patient and clinician. In intermediate risk patient with a first negative surveillance cystoscopy, subsequent cystoscopy should be performed with cytology every 3-6 months for 2 years, then 6-12 months for years 3-4 and then annually thereafter. Lastly, for high risk patients, subsequent cystoscopy with cytology should be performed every 3-4 months for 2 years, then 6 months for years 3-4, and then annually thereafter.
Risk stratification of NMIBC according to recent AUA guidelines:
Presented By: Sam Chang, MD, Vanderbilt University Medical Center, Nashville, TN
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre
Twitter: @GoldbergHanan
at the 2017 Bladder Cancer Academy - June 9 - 10 - Schaumburg, Illinois, USA


