Dr. Hamdy began his talk by discussing the history of prostate cancer prevalence and the emergence of PSA screening. The ProtecT trial, carried out between 1999-2008 over 9 centers, was the largest randomized controlled trial of 82,429 men comparing active monitoring, surgery, and radiotherapy for PSA-detected localized prostate cancer. Additionally, the CAP trial, carried out between 2001-2009 over 573 general practices, randomly assigned a total of 408,825 patients to a PSA testing intervention (as laid out by the ProtecT study) or the comparison arm following standard NHS management. So what does this vast amount of data tell us?
- The risk of prostate cancer over an average of 10 years is very low (1%); most PSA-detected and localized prostate cancers grow very slowly
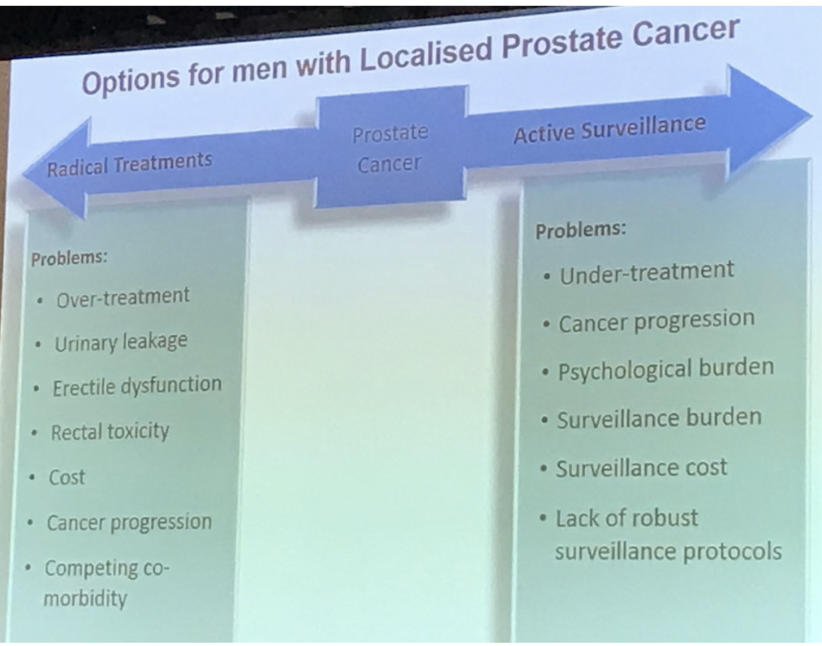
- Surgery and radiotherapy reduce the risk of cancer progression and spread, but cause bothersome urinary sexual, and bowel symptoms (Fig. 1 & 2)
- Staying on active monitoring avoids side effects from treatment, but there is an increased risk of cancer progression and spread (Fig. 1)
- Risk stratification at diagnosis is inaccurate and may be improved by pre-biopsy imaging, targeting, and genomics
- Patients over 65y benefit from radical treatments
- Longer follow up ranging from 5-10 years is essential in ProtecT to provide data about the advantages and disadvantages between short-term radical treatments, risk of disease progression, and long-term survival
- At a median of 10 years for the CAP study, a low-intensity screening intervention (single PSA test) had no discernible effect on PCa-specific mortality
- Did not detect some lethal cancers
- The current diagnostic pathway of PSA-testing a TRUS guided biopsies is inappropriate, no longer suitable, and must evolve to targeting clinically important prostate cancer

Figure 2: Sexual function (top left), urinary continence (top right), and bowel function (bottom)

Figure 3: Problems with treatment options for men with localized prostate cancer
As the lecture continued, Dr. Hamdy stressed the importance of better screening modalities to increase quality care or avoid overtreatment. In unpublished data collected from Oxford, in 197 patients who showed disease progression, 51% of these men had a Gleason score of 3+3. Additionally, in 1,446 patients who did not show disease progression, 2% had a ≥8 Gleason score. There is an obvious disparity between these ranking systems and the true predictive nature of the disease. Similarly, Dr. Hamdy discussed another study that detected 781 men for management through PSA screening, but only 27 of these patients had a need for treatment. Diagnostic screening methods need to improve.
As the session neared its conclusion, Dr. Hamdy offered two apparent solutions to increase patient care for radical treatment: partial gland ablation and near-infrared fluorescence with indocyanine green (NIR ICG). Partial gland ablation is actually prevalent in other parts of the world and has shown to be particularly effective. A study conducted in the UK between 2015 and 2017, known as the PART study, has begun to confirm the viability of partial gland ablation, but the results are still unpublished. In a more technical stance, Dr. Hamdy discussed advancements being made at the University of Oxford which allows for the simultaneous viewing of the visual field and the fluorescence (Fig. 4). The PSMA minibody is used to localize the fluorescent molecules to the cancerous tissue after preoperative injection. This technology has the capability of making partial gland ablation or other radical procedures much safer for the patient.
Figure 4: An example of the novel ICG NIR fluorescence technology with visual field overlay (far right)

Presented by: Freddie Hamdy, University of Oxford, Oxford, United Kingdo
Written By: Zachary Valley, Department of Urology, University of California-Irvine, Twitter: @ZacharyAValley at the 73rd Canadian Urological Association Annual Meeting - June 23 - 26, 2018 - Halifax, Nova Scotia


