Unfortunately, with the time course of advanced prostate cancer, many of our patients are elderly. The decision to treat these patients with ARAT’s can be tough, and a discussion between active treatment and watchful waiting is warranted. However, a direct comparison of these two agents in the elderly population has not been reported – if the decision to treat is made, which agent is preferred? (assuming they do not have a contraindication to either agent)
It should be noted that metastatic prostate cancer is more common in older men. Older men often have increasing comorbidities, altered pharmacokinetics, and increased vulnerability to adverse events. In prior studies of men >= 75, the incidence of drug-related AEs are similar and treatment outcomes are similar.
In this retrospective series from British Columbia, the authors address this question in men older than 80. They specifically focused on men ≥80 years of age who received abiraterone (ABI) or enzalutamide (ENZA) for first-line treatment of mCRPC between July 2009 and September 2016. Primary goals were to assess oncologic outcomes - prostate-specific antigen (PSA) response rate (PSA50) (decrease of ≥50% from baseline), time to first progression (TTP) (PSA [PCWG3 criteria], radiographic or clinical progression), and overall survival (OS).
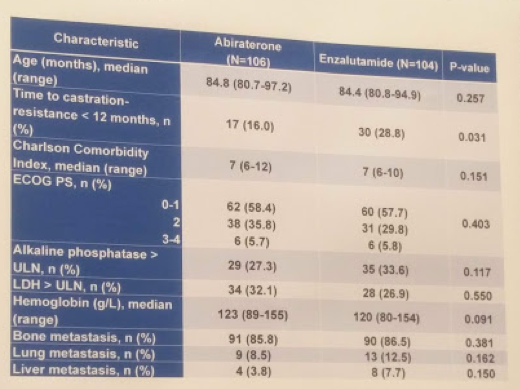
During this time frame, they identified 106 patients in the ABI cohort and 104 in the ENZA cohort. When comparing the two groups, most patient and disease characteristics were similar, except for time to castration resistance - a higher proportion of patients in the ENZA cohort had a time to castration resistance <12 months. Baseline characteristics are listed below:
In terms of outcomes, PSA50 was 43.4 % for ABI vs. 77.9 % for ENZA (p˂0.001). The median TTP was 4.7 months for ABI vs. 8.0 months for ENZA (hazard ratio [HR] 1.52; 95% confidence interval [CI ]1.12-2.08). Median OS was 13.2 months for ABI vs. 18.7 months for ENZA (HR 1.20; 95% CI 0.89-1.63).
They did look at predictors of time to progression. Following a univariate analysis, they did an MV analysis (using those factors that were significant on univariate analysis), and found that abiraterone use was associated with faster time to progression (HR 1.76, p<0.001). Other variables included serum alkaline phosphatase, hemoglobin, time to castration resistance, and Charlson comorbidity index.
In terms of tolerability, treatment was discontinued for toxicity in 8.5% of patients for ABI and 13.5% for ENZA (p=0.276). At least one dose reduction due to toxicity was required for 7.5% of patients for ABI vs. 29.8% for ENZA (p˂0.001).
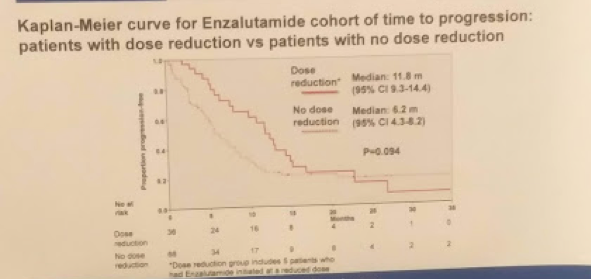
Specifically, in men in the ENZA cohort who had a dose reduction, the median TTP was 11.8 months vs. 6.2 months for those without (HR 0.65; 95% CI 0.40-1.08, p = 0.094) – suggesting dose reduction allowed for longer response. Apparently, the starting dose may be too strong for some patients and a lower dose will have the same pharmacokinetic properties.
Based on this, it would appear that in men older than 80 who are eligible for treatment for cM0 CRPC (and are willing to be treated actively), the PSA50 and TTP were superior for the ENZA cohort compared to the ABI cohort – but this did not translate to improved overall survival (though this may have been underpowered, trending towards significance). Dose reductions in the ENZA cohort did not negatively affect TTP, suggesting this is still an effective way of keeping patients on treatment – though, without knowing the details, confounders may exist.
Presented by: Daniel J.Khalaf, Fellow, BC Cancer Agency Vancouver Centre, Vancouver, Canada
Co-Authors: Kevin Zou, Werner Struss, Bernhard Eigl, Christian K Kollmannsberger, Daygen Finch, Krista Noonan, Joanna Vergidis, Muhammad Zulfiqar, Kim N Chi
Author Information:
1. Department of Medical Oncology, BC Cancer Agency - Vancouver Centre, Vancouver, BC, Canada;
2. Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada
3. Department of Urology, University of British Columbia, Vancouver, BC, Canada
4. Department of Medical Oncology, BC Cancer Agency - CSI, Kelowna, BC, Canada
5. Department of Medical Oncology, BC Cancer agency - Surrey, Surrey, BC, Canada
6. Department of Medical Oncology, BC Cancer Agency - Victoria, Victoria, BC, Canada
7. Department of Medical Oncology, BC Cancer Agency - Abbotsford, Abbotsford, BC, Canada
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 73rd Canadian Urological Association Annual Meeting - June 23 - 26, 2018 - Halifax, Nova Scotia


