Dr. Breau began with two statements that according to him, everyone can agree upon:
- Perioperative chemotherapy benefits some patient with UTUC, but harms most of the patients.
- Neoadjuvant chemotherapy overtreats many patients.
The results of the trial showed a clear benefit to the adjuvant chemotherapy arm in disease-free survival, with a hazard ratio of 0.49 (0.31-0.76), p=0.001 (Figure 1). Although a separation in the overall survival curve was demonstrated, the difference was not statistically significant, most probably due to the early analysis (Figure 2). As expected, the adverse effects in the adjuvant chemotherapy arm were significantly higher than in the surveillance arm (Figure 3).
Figure 1 – Disease-Free Survival in the Pout Trial Demonstrating a Clear Advantage to Adjuvant Chemotherapy Over Surveillance:

Figure 2 – Overall Survival in the Pout Trial Demonstrating a Trend Favoring Adjuvant Chemotherapy, But No Statistical Significance:
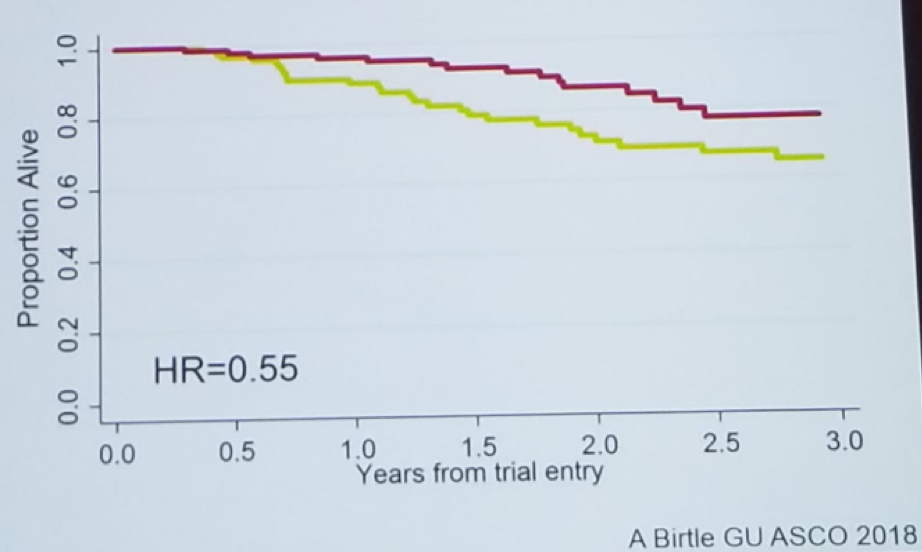
Figure 3 – Adverse Events in the POUT Trial:

It is important to remember that the POUT trial included patients with a >=pT2 pNany disease. However, when assessing pathology reports of patients who undergo radical nephroureterectomy in 15 different centers (3544 patients), 16% had the pT2 disease, 35% had pT0,pTa, or CIS disease, 23% had pT1 disease, and only 25% had a pT3-T4 disease.2 In other words, 58% of patients that usually undergo radical nephroureterectomy would have been excluded from participating in the POUT trial. The 5- year cancer-specific survival without perioperative chemotherapy for T0-Ta-CIS, T1, T2, T3-4 are 88%, 80%, 71%, and 46%, respectively. That means that most patients undergoing radical nephroureterectomy have excellent cancer-specific survival without the addition of chemotherapy.
These data show that the administration of neoadjuvant chemotherapy will overtreat patients with low stage disease, while adjuvant chemotherapy will undertreat patients with high stage disease (due to a loss in renal function, and inability to administer chemotherapy). According to our best estimates, 65% of patients will be cured by surgery alone, 50% of patients are eligible for neoadjuvant chemotherapy, and 25% are eligible for adjuvant chemotherapy. In a simple calculation, as shown in figure 4, when comparing adjuvant to neoadjuvant chemotherapy, the number of cured patients is almost identical, but the percentage of patients receiving chemotherapy is considerably higher in the neoadjuvant group, with its associated grade 3-4 toxicity. These data show that 65 additional patients need neoadjuvant chemotherapy to save a maximum of 1 life vs. adjuvant chemotherapy. According to Dr. Breau, this comparison makes the choice between neoadjuvant and adjuvant chemotherapy quite simple, clearly favoring the adjuvant way.
Figure 4 – Theoretical Comparison of Adjuvant and Neoadjuvant Chemotherapy in UTUC:

In summary, perioperative chemotherapy benefits some patients but harms most of them. Neoadjuvant chemotherapy administered to all overtreats too many patients. The likely best approach for deciding who to give neoadjuvant chemotherapy is a careful preoperative selection of patients with advanced clinical stage disease, and poor pre-treatment renal function (GFR<75 ml/min).
Presented by: Rodney H. Breau, Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Surgical Oncologist, an Assistant Professor of Urology at The University of Ottawa, and an Associate Scientist at the Ottawa Hospital Research Institute.
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter: @GoldbergHanan at the CUOS – Canadian Uro-Oncology Summit 2019, #CUOS19 January 10-12, 2019 Westin Harbour Castle, Toronto, Ontario, Canada
References:
1. Birtle A et al. GU ASCO 2018
2. Ploussard G et al. Eur Urol 2015
Further Related Content:
High-grade Upper Tract Urothelial Carcinoma – Neoadjuvant Chemotherapy


