He began his discussion stating the enormous changes that have occurred in the last 15 years in the field of advanced prostate cancer. Many new treatments have been added that extend survival. These include chemotherapy (docetaxel, cabazitaxel), Sipuleucel T, androgen axis inhibitors (abiraterone, enzalutamide, apalutamide) and Radium 223. Additionally, supportive agents have been added including bone-targeted therapies (denosumab and zoledronic acid).
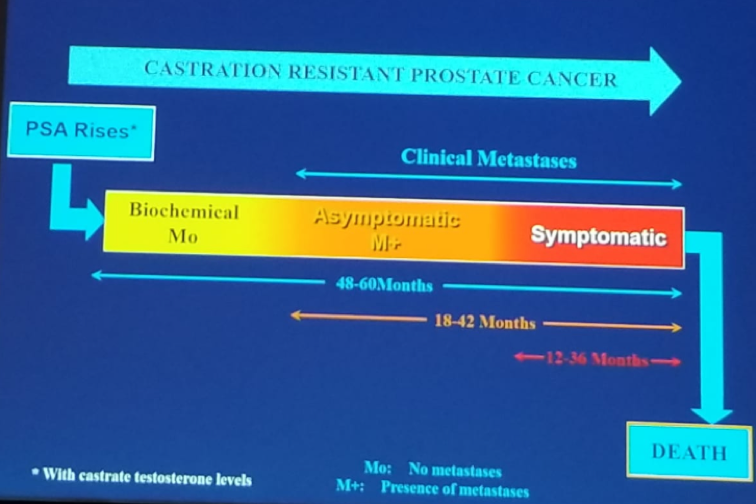
CRPC disease is defined when a patient has serum castration levels of testosterone, with three executive rises of PSA, one week apart, resulting in two 50% increases over the nadir, with a PSA >2 ng/ml. Lastly, there is also the progression of osseous lesions: progression or appearance of two or more lesions on bone scan, or soft tissue lesions using the RECIST criteria together with nodes >=2 cm in diameter. Figure 1 demonstrates the progression phases of advanced prostate cancer.
Figure 1 – Progression of Advanced Prostate Cancer:
There have been several trials assessing various agents in the setting of CRPC. The first was the COU-AA-302 phase 3 double-blind prospective randomized controlled trial, that analyzed the effects of abiraterone and prednisone compared to placebo and prednisone in chemo-naive CRPC patients.1 The trial showed an overall survival benefit for abiraterone with a hazard ratio of 0.7 (95% CI 0.66-0.95, p=0.02), and with a survival advantage of 5 months (35.3 vs. 30.1 months). Another study was the PREVAIL – a phase 3 trial of enzalutamide given to metastatic CRPC patients after progression on androgen deprivation therapy (ADT).2 Compared to placebo, Enzalutamide was demonstrated to have improved radiographic progression-free survival (HR 0.186, 95% CI 0.15-0.23, p<0.0001) and overall survival (HR 0.7, 95% CI 0.6-0.084, p<0.0001).
Next, Dr. Fleshner discussed the pros and cons of treating a patient with nmCRPC. The proposed advantages include the prevention or delay of metastatic disease development, prevention of symptoms, and a questionable benefit in overall survival. The disadvantages include the adverse effects associated with the early administration of these agents, the higher cost associated with treatment, and the fact that overall survival might not improve.
There have been two trials assessing these treatments in the specific setting of nmCRPC. The first is the SPARTAN prospective randomized controlled trial, comparing placebo to Apalutamide. 3 Apalutamide was shown to have 73% risk reduction of distant progression (HR 0.27, 95% CI 0.22-0.34, p<0.0001), and improved overall survival, although it was not statistically significant (HR 0.7, 95% CI 0.47-1.04, p=0.07). The second study was the PROSPER prospective randomized controlled trial, comparing enzalutamide to placebo in the setting of nmCRPC.4 Enzalutamide was shown to have improved metastases-free survival (HR 0.29, 95% CI 0.24-0.36, p<0.0001) with 36.6 vs. 14.7 months. Like the SPARTAN trial, the overall survival was improved with enzalutamide, but this was not statistically significant (HR 0.8, 95% ci 0.58-1.09, P=0.1519). Lastly, it is important to mention that the results of another trial, examining another agent in the same setting of nmCRPC, are due to be published in the next GU-ASCO meeting in February 2019. This is the: Efficacy and Safety Study of Darolutamide (ODM-201) in Men with High-risk Non-metastatic Castration-resistant Prostate Cancer (ARAMIS) (NCT02200614), and we eagerly await its results.
The adverse effects of the three commonly used medications for nmCRPC include:
- Abiraterone - hyperkalemia, hypertension, elevation of liver enzymes, and fluid retention
- Enzalutamide – hypertension, and seizures
- Apalutamide – Hypertension, rash, and hypothyroidism
Concluding his talk, Dr. Fleshner reiterated that nmCRPC trials demonstrate an impressive delay in time to metastases. Overall survival trends are generally encouraging, suggesting that earlier therapy leads to a better outcome, with reported toxicity that seems manageable.
Presented by: Neil Fleshner, MD, MPH, FRCSC, Chair and Professor at the Division of Urology, University of Toronto, Princess Margaret Cancer Center, Toronto, Canada
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter: @GoldbergHanan at the CUOS – Canadian Uro-Oncology Summit 2019, #CUOS19 January 10-12, 2019 Westin Harbour Castle, Toronto, Ontario, Canada
References:
1. Ryan C. et al. NEJM 2013
2. Beer TM et al. NEJM 2014
3. Smith MR et al. NEJM 2018
4. Hussein M. et al. NEJM 2018


