In this abstract, the authors highlight the initial data from the PART trial (Partial prostate Ablation versus Radical prostatectomy (RP)), the first phase III study to compare PA (partial ablation) and RP in intermediate risk prostate cancer. PART is a prospective, multi-centre, parallel group randomized controlled trial (RCT) to assess the clinical effectiveness and cost-utility of partial ablation using HIFU or RP in patients with intermediate risk, unilateral clinically localized prostate cancer. They hypothesize that PA can achieve organ preservation, reduce side effects, maintain good voiding and sexual function, without compromise to oncological outcomes. The main multi-center RCT will be soon initiated based on these results.
Eligibility:
• Patients diagnosed with intermediate risk, unilateral clinically localized prostate cancer, fit for either RP or HIFU. Intermediate risk was defined by Gleason grade 4 (Gleason score 3+4 of 4+3) and/or >4 mm cancer core length irrespective of Gleason, PSA <= 20, <= cT2b disease.
• mpMRI and targeted biopsy or template guided biopsies were required before randomization to either RP or PA
• Target accrual for the feasibility phase was 80 patients over 18 months
• Follow-up involved regular PSA levels and, in the PA arm, MRI and targeted biopsies of any suspicious areas – no specific details re: timing of MRI and repeat biopsy. All will have mpMRI prior to biopsy.
• Quality of life data were measured at 6 weeks and 3 monthly intervals.
Table 1 summarizes recruitment data.

As seen above, of 242 patients screened, 56 consented to the randomization. The study is on intent-to-treat basis. Three of the patients who consented to RP changed allocation – 1 underwent HIFU, 1 Active surveillance, 1 radiotherapy.
They presented slightly updated numbers now, with 70 men randomized. Of note, still, 6 of the 34 men randomized to RP refused RP.
Table 2 describes patient baseline characteristics currently submitted
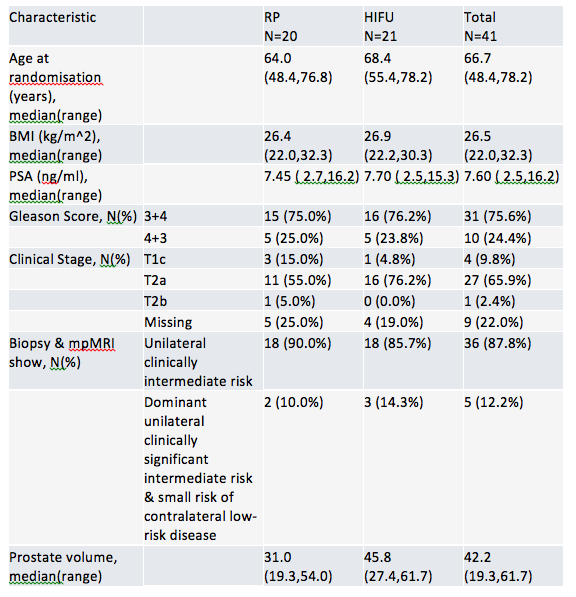
Groups were evenly matched. Based on this ability to recruit, the study will proceed – expect total recruitment of N=600-800. The results promise to be exciting and add to our growing knowledge.
Dr. Baniel (Israel) did comment, appropriately, that the risk of undiagnosed contralateral disease in men with Gleason 4+3 pathology is approximately 50% - is it appropriate to consider them for focal therapy? Dr. Leslie acknowledged this and explained that the study will also end up assessing if their current imaging and evaluation technology is sufficient to correctly identify patients with truly focal disease.
Speaker: Tom Leslie
Co-Author(s): Elliott D., Le Conte S., Brewster S., Sooriakumaran P., Bryant R., Dudderidge T., Rosario D., Catto J., Hindley R., Emberton M., Ahmed H., Donovan J., Hamdy F.
Institution(s):
1. Oxford University - Churchill Hospital, Dept. of Urology, Oxford, United Kingdom
2. Churchill Hospital, Dept. of Urology, Oxford, United Kingdom
3. University of Bristol, Dept. of Social and Community Medicine, Bristol, United Kingdom
4. University Hospital Southampton NHS Foundation Trust, Dept. of Urology, Southampton, United Kingdom
5. Sheffield Teaching Hospitals, Dept. of Urology, Sheffield, United Kingdom
6. Basingstoke and North Hampshire Hospital, Dept. of Urology, Basingstoke, United Kingdom
7. University College London Hospital, Dept. of Urology, London, United Kingdom
Written By: Thenappan Chandrasekar, MD, Clinical Fellow, University of Toronto
Twitter: @tchandra_uromd
at the #EAU17 - March 24-28, 2017- London, England


