For this study, a total of 426 patients who underwent radical nephroureterectomy at five medical centers between January 1995 and April 2017 were examined retrospectively. Of the 426 patients, 234 were treated for a high-risk disease (stages cT3–4 or locally advanced [cN+] disease) with or without neoadjuvant chemotherapy. Chemotherapy regimens were selected based on eligibility of cisplatin. The authors retrospectively evaluated post-therapy pathological downstaging, lymphovascular invasion, and prognosis stratified by neoadjuvant chemotherapy use. Multivariate Cox regression analysis was performed for independent factors for prognosis.
Among the 234 patients included, 101 received neoadjuvant chemotherapy and 133 did not. The regimens in the neoadjuvant chemotherapy group included gemcitabine and carboplatin (75%), and gemcitabine and cisplatin (21%). Pathological downstaging of the primary tumor and lymphovascular invasion were significantly improved in the neoadjuvant chemotherapy than in the control groups. Neoadjuvant chemotherapy for locally advanced UTUC significantly prolonged recurrence-free and cancer-specific survival.
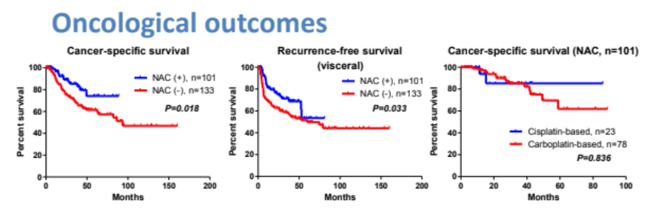
Multivariate Cox regression analysis using an inverse probability of treatment weighted method showed that neoadjuvant chemotherapy was selected as an independent predictor for prolonged recurrence-free and cancer-specific survival. However, the influence of neoadjuvant chemotherapy on overall survival was not statistically significant.
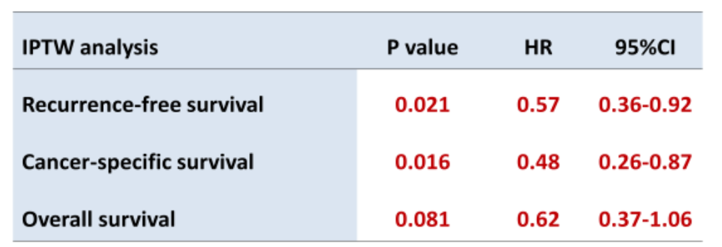
The authors concluded that platinum-based neoadjuvant chemotherapy for locally advanced UTUC potentially improves oncological outcomes in their multicenter study. However, they do caution that further prospective studies are needed to clarify the clinical benefit of neoadjuvant chemotherapy for locally advanced UTUC.
Presented by: Yuka Kubota, Hirosaki University Graduate School of Medicine, Hirosaki, Japan
Co-Authors: Hatakeyama S, Soma O, Matsumoto T, Kusaka A, Hosogoe S, Hamano I, Tobisawa Y, Yoneyama T, Yamamoto H, Imai A, Yoneyama T, Hashimoto Y, Koie T, Ito H, Yoshikawa K, Sasaki A, Kawaguchi T, Ohyama C
References:
1. Birtle AJ, Chester JD, Jones R, et al. Results of POUT: A phase III randomized trial of perioperative chemotherapy versus surveillance in upper tract urothelial cancer (UTUC). J Clin Oncol 36, no. 6_suppl (February 2018) 407.
Written by: Zachary Klaassen, MD, Urologic Oncology Fellow, University of Toronto, Princess Margaret Cancer Centre, twitter: @zklaassen_md at the 2018 European Association of Urology Meeting EAU18, 16-20 March, 2018 Copenhagen, Denmark
Read More:
Results of POUT – A Phase III Randomized Trial of Peri-Operative Chemotherapy Versus Surveillance in Upper Tract Urothelial Cancerf Peri-Operative Chemotherapy Versus Surveillance in Upper Tract Urothelial Cancer


