However, patients with non-metastatic CRPC (nmCRPC) had no standard of care therapy. As a result, these patients are often managed expectantly. Unfortunately, most of these men eventually progress to metastatic disease, at varying rates. A recent retrospective series identified that among nmCRPC men, nearly 60% developed metastatic disease during the first 5-years, with most of the metastasis occurring within the first 3 years. [3]
At the EAU 2018 meeting in Copenhagen, Dr. Cora Sternberg presented a poster of a prospective randomized phase 3 double blind, placebo controlled multinational clinical trial (NCT02003924) specifically assessing the treatment of men who were nmCRPC patients. The PROSPER study, initially presented at GU-ASCO 2018 in San-Francisco, specifically assesses the effect enzalutamide has in these patients. For the purpose of this trial, the following patients were enrolled:
- Men with asymptomatic M0 CRPC
- With PSA doubling time ≤ 10 months
- PSA ≥ 2 ng/mL at screening with testosterone < 50 ng/dl
All men continued ADT (medical or surgical castration) and were randomized 2:1 to enzalutamide 160 mg with ADT or placebo + ADT (Figure 1). The primary endpoint was metastasis free survival (MFS) (defined as the time from randomization to radiographic progression or death on study).
The secondary endpoints included:
- Time PSA progression
- Time to use of new antineoplastic therapy
- Time to PSA progression
- PSA response
- Overall survival (OS)
- Safety
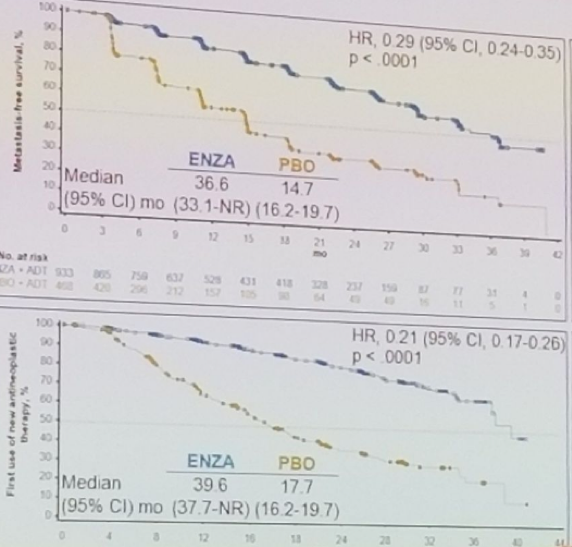
- Risk of MFS (hazard ratio [HR] 0.29; 95% confidence interval [CI] 0.24-0.35; P < .0001)
- Time to first use of new antineoplastic therapy (HR 0.21; 95% CI 0.17-0.26; P < .0001)
- Time to PSA progression (HR 0.07; 95% CI 0.05-0.08; P < .0001)
In the first interim analysis of OS there was a trend in favor of enzalutamide (HR 0.80; 95% CI 0.58-1.09; P = .1519).
A significantly greater proportion of men had confirmed PSA responses with enzalutamide compared to placebo with:
- ≥ 50% decline (76.3% vs 2.4%; P < .0001)
- ≥ 90% decline (55.9% vs 0.4%; P < .0001)
- Undetectable levels below the limit of quantification (9.6% vs 0%; P < .0001)
- Any grade: 87% vs 77%
- Grade ≥ 3: 31% vs 23%
- Grade > 5: 3.4% vs 0.6%
In conclusion, in men with nmCRPC and rapidly rising PSA, treatment with enzalutamide resulted in a clinically meaningful and statistically significant reduction in the risk of developing M1 CRPC, with a 71% relative risk reduction of metastasis or death compared to placebo. There was a significantly greater PSA response with enzalutamide compared to placebo. A significant increase in the median time to PSA progression, and time to use of new antineoplastic drug was noted in the enzalutamide arm. Furthermore, there was a positive trend for OS with enzalutamide. Lastly, AEs were consistent with the established safety profile of enzalutamide, which was generally well tolerated.
Figure 1 – PROSPER study design:
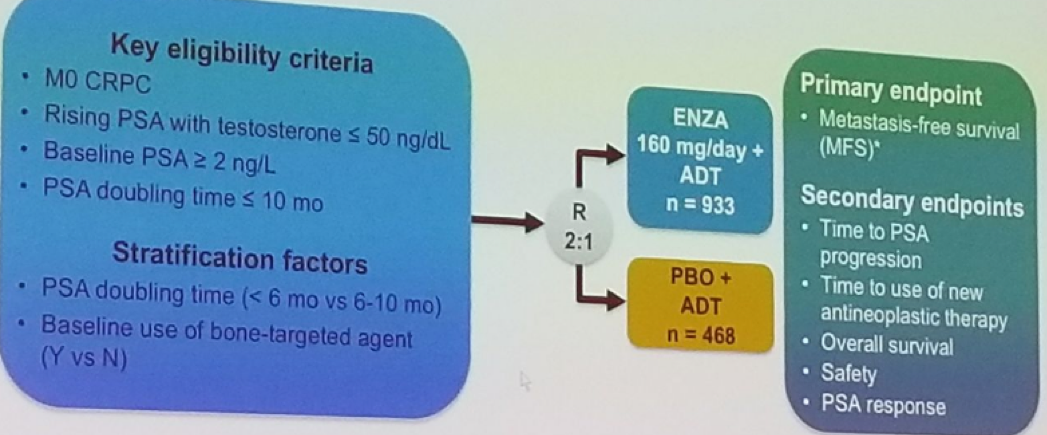
Figure 2 – PROSPER results - primary and secondary endpoints:
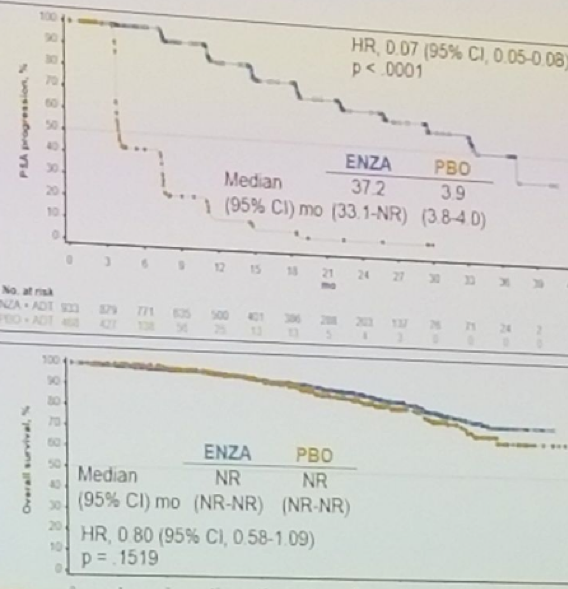
Watch: Changing the Standard of Care in the M0 CRPC Patient: PROSPER - A Conversation with Cora Sternberg
View Results from PROSPER - Poster #604
Presented by: Cora Sternberg, San Camillo and Forlanini Hospitals, Dept. of Medical Oncology, Rome, Italy
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, twitter: @GoldbergHanan at the 2018 European Association of Urology Meeting EAU18, 16-20 March, 2018 Copenhagen, Denmark
References:
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
3. Moreira DM, Freedland SJ, et al. Predictors of Time to Metastasis in Castration-resistant Prostate Cancer. Urology. 2016 Oct;96:171-176. doi: 10.1016/j.urology.2016.06.011. Epub 2016 Jun 16.
More Related Content:
Patient-Reported Outcome Measures in Men with Non-Metastatic Castration-Resistant Prostate Cancer: Baseline Data from the PROSPER Trial
Watch: Meeting an Unmet Need in the Non-Metastatic Castration Resistant Prostate Cancer Patient Population - Maha Hussain
Watch: Discussion on PROSPER: Safety and Efficacy Study of Enzalutamide in Patients with nmCRPC - Thomas Keane
First Presentation - PROSPER: Safety and Efficacy Study of Enzalutamide in Patients With Nonmetastatic Castration-Resistant Prostate Cancer (nmCRPC)
Review of First Presentation: SPARTAN and PROSPER


