According to Dr. Shariat, there are several reasons to consider sequencing:
- Risk assessment: what is the probability of disease?
- Prevention: is the disease risk modifiable?
- Prognosis: does germline alteration have an impact on prognosis?
- Treatment selection: are certain treatments more or less effective in the setting of specific mutations?
- Familial risk and testing: is there a need for testing of family members?
Indeed, the genetic predisposition to malignancy is multifaceted:
Hereditary urothelial carcinoma makes up ~15% of upper tract urothelial carcinomas and ~5% of bladder urothelial carcinomas. It is usually due to a single inherited genetic mutation, greatly increasing the lifetime risk of disease (ie. Lynch syndrome). Familial urothelial carcinoma makes up ~10% of cases and does share some features with hereditary cancer. Importantly, familial disease has no detectable mutation that can be identified and disease risk may also be combined with environmental risk. Sporadic urothelial carcinoma makes up ~80% of cases and exact causes are unknown except for the increased risk with tobacco smoking. There are no features of hereditary or familial cancers and there is no increased risk for close family members.
The hereditary nonpolyposis colorectal cancer (HNPCC) tumor spectrum includes germline mutations in five DNA mismatch repair genes, including MLH1, MSH2, MSH6, PMS2, and/or EPCAM. For these patients, the cumulative lifetime risk of developing urothelial carcinoma is 1-28%. Microsatellite instability is frequent in upper tract urothelial carcinoma (~25%) and less common in bladder urothelial carcinoma (~3%). Selection for hereditary screening at the first medical interview is as follows, as adapted from the EAU guidelines:1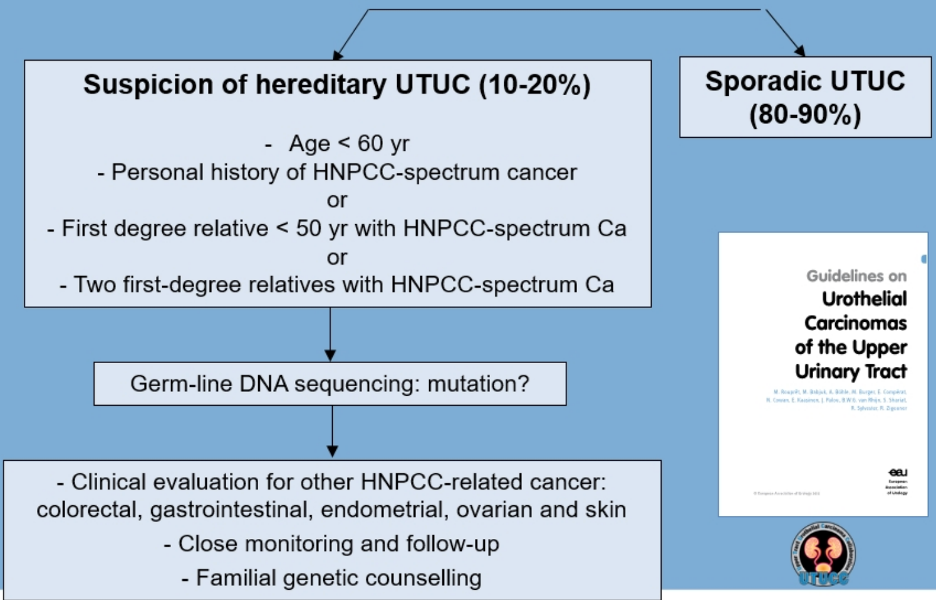
A recent paper from Hassler et al.2 in European Urology has further highlighted the molecular characterization of upper tract urothelial carcinoma:
DNA damage response pathways are implicated in several cancers. Healthy cells have a plethora of DDR processes that protect their DNA, however, genetic predisposition arises when one DNA repair process is compromised. Progression from this precancerous state to ongoing proliferation requires the downregulation of these DDR processes, facilitating persistent genomic instability. DDR pathway alterations are common in urothelial carcinoma, potentially opening the door for PARP inhibitor therapy. To summarize, comprehensive molecular characterization of bladder urothelial carcinoma has shown that these tumors have multiple mutational drivers, a large mutational load, multi-targeted therapies may be required, significant intratumoral heterogeneity and resistance to treatment is common and may occur early. Indeed, 69% of tumors harbor potential therapeutic targets! A summary of the comprehensive molecular characterization of muscle-invasive bladder cancer is as follows:3
Biomarker-driven therapy has several criteria based on molecular characteristics, including patients that are most likely to respond and those that may benefit from faster and more conclusive answers. In the old model, there were large populations with low efficacy with unnecessary side effects, whereas in the new model there are small populations with relevant molecular defects and all patients have the potential to respond. Bringing the ideal marker into clinical practice has several requirements according to Dr. Shariat:
- Easier: performed easily and promptly in a clinical environment
- Better: the biomarker adds clinical value
- Faster: available in an efficient and timely manner
- Cheaper: cost-effective
Selective FGFR inhibitors have emerged in urothelial cancer, including erdafitinib. In the BLC2001 phase 2 trial, 99 patients with FGFR alterations were treated with a median of five cycles of erdafitinib.4 Among these patients, 43% had received at least two previous courses of treatment, 79% had visceral metastases, and 53% had a creatinine clearance of < 60 ml/min. The rate of confirmed response to erdafitinib therapy was 40% (3% with a complete response and 37% with a partial response), and among 22 patients who had undergone previous immunotherapy, the confirmed response rate was 59%. The median duration of progression-free survival was 5.5 months, and the median duration of overall survival was 13.8 months. Furthermore, 76% of patients treated with erdafitinib had a reduction in the sum of target-lesion diameters.
Dr. Shariat provided the following framework for multigene testing for urothelial cancer, highlighting the needs for genetic testing among all late-stage and metastatic urothelial carcinoma patients: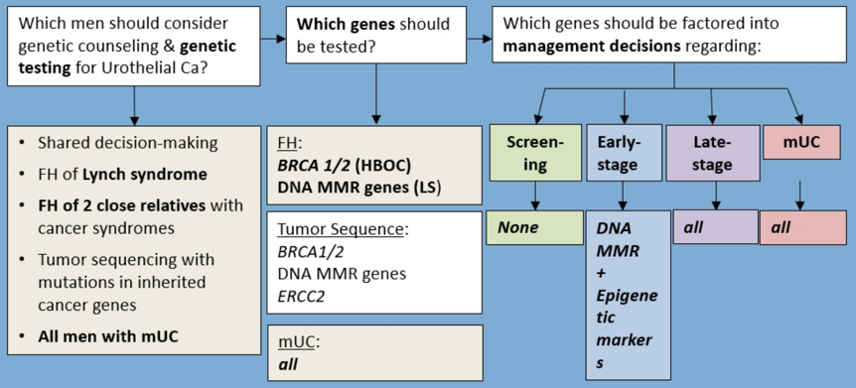
Dr. Shariat concluded this high-level presentation discussing sequencing of urothelial patients with the following concluding remarks:
- Cancer biology is the primary mediator of morbidity and mortality, and only better diagnosis and therapies will reduce this burden
- NextGen sequencing and integrated multiplatform analyses inform biology, facilitating a personalized therapy approach and should be an integral part of tumor boards
- The delineation of the genomic landscape and molecular subtypes will accelerate biomarker and drug development
- We must rely on biology to inform our clinical practice
Some final thoughts from Dr. Shariat include (i) the high prevalence of mutations suggests all patients with metastatic urothelial carcinoma should be offered testing, (ii) the most critical urothelial cancer genes today are MMR, FGFR, DNA repair, (iii) new tests are commercially available, so we need to know the performance characteristics, and (iv) there are key implications for patients (treatment of metastatic disease), and family (prevention, screening).
Presented by: Shahrokh F. Shariat, MD, Medical University of Vienna, Vienna, Austria
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the Virtual 2020 EAU Annual Meeting #EAU20, July 17-19, 2020.
References:
- Roupret M, Babjuk M, Capoun O, et al. European Association of Urology Guidelines on Upper Tract Urothelial Carcinoma: 2020 Update. Eur Urol 2020 Jun 24:S0302-2838(20)30427-9.
- Hassler MR, Bray F, Catto JWF, et al. Molecular Characterization of Upper Tract Urothelial Carcinoma in the Era of Next-generation Sequencing: A Systematic Review of the Current Literature. Eur Urol 2020 Jun 20;S0302-2838.
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017 Oct 19;171(3):540-556.
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2019 Jul 25;381(4):338-348.


