(UroToday.com) At the European Association of Urology (EAU) Virtual 2020 meeting, Olivier Rouviere, MD, PhD, concluded the plenary session on “Modern prostate cancer imaging in daily practice,” by discussing the impact of artificial intelligence and prostate cancer imaging. Dr. Rouviere notes that machine learning entails training a machine to discriminate prostate cancer on magnetic resonance (MR) images using pattern recognition and statistical tools. The principles and algorithm of machine learning are as follows:
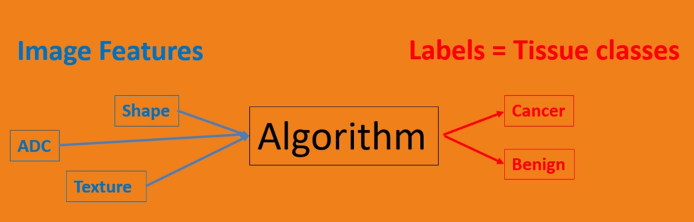
Dr. Rouviere highlighted that the starting point is crucial, what he calls the “ground truth”, in that there must be a carefully labeled training dataset. Handcrafted features are prespecified imaging features chosen by experts based on prior knowledge and include (i) ADC, (ii) perfusion parameters (wash-in rate, wash-out rate, TTP), (iii) texture parameters, and (iv) shape and size. Next, the classifier predicts the tissue classes based on these imaging features, utilizing linear/logistic regression, support vector machines, random forests, and naïve Bayes classification.
Non-handcrafted features include the processing of the raw data, which is part of the learning itself according to Dr. Rouviere. The algorithm then learns to extract its own features to optimize the prediction labels. An example of this is deep learning methods using artificial neural networks. Deep learning allows the potential to “see” new features, as well as the need for large training databases.
The big issue, according to Dr. Rouviere, is a generalization. Indeed, good performance of the training database does not equal good performance on an independent database. Overfitting may generate perfect performance on the training database, but poor performance on external validation. Robustness of MR quantification is also important, as there may be large variation of MR features across vendors, machines, and different sites. Currently, there is an extensive effort for standardization led by the Quantitative Imaging Biomarkers Alliance. External validation is key, including using different patients, scanners, institutions, and vendors.
Over the last 10 years there has been a large body of literature in the field, but very few systems have undergone external validation, notes Dr. Rouviere. One platform is the Watson ElementaryTM, which is commercially available software, allows co-registration of pulse-sequence imaging, and denotes a ‘Malignancy Attention Index’ based on features from T2-weighted images, diffusion-weighted images, and Dynamic Contrast Enhanced (DCE) imaging. In an evaluation of 45 patients undergoing multi-parametric magnetic resonance imaging (mpMRI) scans, Roethke et al.1 reported a sensitivity of 85.7% (with 95% CI of 65.4-95.0), a specificity of 87.5% (with 95% CI of 69.0-95.7) and a diagnostic accuracy of 86.7% (with 95% CI of 73.8-93.8) for detection of prostate cancer. Additionally, the automated analysis results corresponded well with the reported diagnostic accuracies by human readers based on the PI-RADS system. Unfortunately, these results were not reproduced at external validation secondary to the exclusion of a substantial number of patients with poor image quality.
A subsequent study assessed the computer-aided diagnosis system’s ability to assist in prostate cancer detection compared with conventional mpMRI interpretation in a dataset acquired from five institutions and tested by nine readers of varying experience level.2 There was a trend towards improved detection of transition zone lesions for less experienced readers (p=0.055). A table of the metrics is as follows from this study: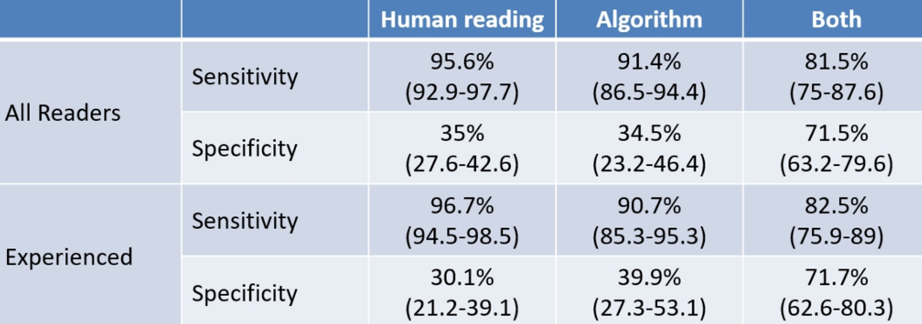
With regard to deep learning, there have been seven studies published in the last three years with promising results, but no true external validation has been performed as of yet.
Dr. Rouviere concluded his presentation with the following take-home messages:
- Conventional machine-learning techniques provided interesting results in the detection of high-grade cancers
- The issue of generalization of results remains a major problem
- For the moment, deep learning techniques have not shown superior results
- There is no doubt that they will in the future, provided there are large training databases available
- The creating of multi-vendor multi-center large databases is needed
Presented by: Olivier Rouviere, MD, PhD, Professor, Rijnstate Hospital Arnhem, Arnhem, The Netherlands
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, twitter: @zklaassen_md at the 35th Annual EAU Congress, 2020 Virtual Program #EAU20, July 17-19, 2020.
References:
- Roethke MC, Kuru TH, Mueller-Wolf MB, et al. Evaluation of an Automated Analysis Tool for Prostate Cancer Prediction Using Multiparametric Magnetic Resonance Imaging. PloS One. 2016 Jul 25;11(7):e0159803.
- Gaur S, Lay N, Harmon SA, et al. Can computer-aided diagnosis assist in the identification of prostate cancer on prostate MRI? A multi-center, multi-reader investigation. Oncotarget 2018 Sep 18;9(73):33804-33817.


