(UroToday.com) As part of the focal therapy for prostate cancer thematic session at the European Association of Urology (EAU) Virtual 2020 meeting, Hashim Ahmed, PhD, FRCS(Urol), from London discussed midterm oncological results of focal cryoablation therapy. Dr. Ahmed notes that we must remember the priorities for our patients, in particular the risks and benefits of treatment for localized prostate cancer. Focal high intensity focused ultrasound (HIFU) and focal cryotherapy are both National Institute for Health and Care Excellence (NICE) approved treatments for localized prostate cancer, as long as there are provisions for prospective data collection. Indeed, there is the High-Intensity Focused Ultrasound Evaluation and Assessment of Treatment (HEAT) registry for HIFU and the ICE registry for cryotherapy.
Focal therapy inclusion criteria include:
- Clinical: Prostate-sepcific antigen (PSA) ≤ 20 ng/mL, radiological ≤ T3aN0M0, and the lesion is in no more than one quadrant on magnetic resonance imaging (MRI)
- Histology: Gleason 7 (4+3 or 3+4), maximal Gleason 4+4 on targeted biopsies provided the lesion is Gleason 7 is permitted, no cancer length limit on Gleason 7, and Gleason 3+3 if >= 6 mm in length and MRI lesion is Prostate Imaging Reporting & Data System (PI-RADS) 3, 4 or 5
- Biopsy: templated or targeted/systematic biopsies
- Contralateral lobe: up to 5 mm of Gleason 3+3 permitted, and MRI PI-RADS score 1, 2 or 3 is permitted
A systematic review from 2014 yielded 9 manuscripts of primary focal prostate cryotherapy and 2 papers for focal salvage treatment (radio-recurrent).1 Among 1,582 primary patients, the biochemical disease free-survival was between 71-93% at 9-70 months follow-up an incontinence rates were 0-3.6% (erectile dysfunction rate: 0-42%). Recently published data from the UK ICE registry included 122 consecutive patients undergoing focal cryotherapy between 2013 and 2016.2 There were 80 (65.6%) patients that had anterior ablation, 23 (19.7%) combined posterior and anterior ablation, and two (1.6%) had posterior ablation alone. The median age was 68.7 years (IQR 64.9-73.8) and preoperative PSA was 10.8ng/ml (IQR 7.8-15.6). Overall, the failure-free survival at 3 years was 90.5% (95% CI 84.2-97.3, and when stratified for the National Comprehensive Cancer Network (NCCNa0 risk group, the 3-year outcomes were 84.7% (95% CI 71.4-100) in high risk and 93.3% (95% CI 86.8-100) in intermediate-risk: 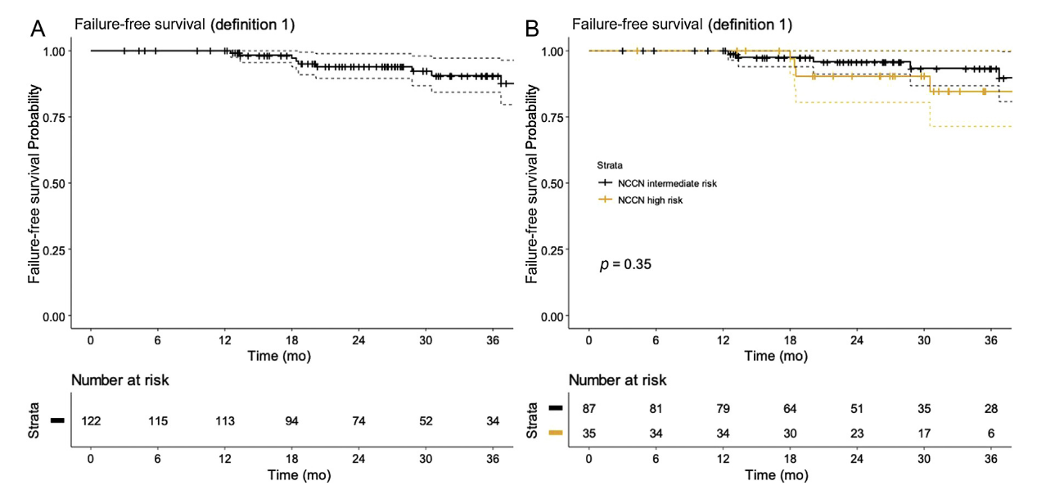
Additionally, there was no incontinence at 12 months (defined as any pad-usage) and the erectile dysfunction rate was 16%. The probability of erectile function return to baseline at 12 months was 85% and 92% at 18 months. Currently, the CHRONOS trial is assessing men eligible for focal or radical therapy and randomizing men in the following fashion: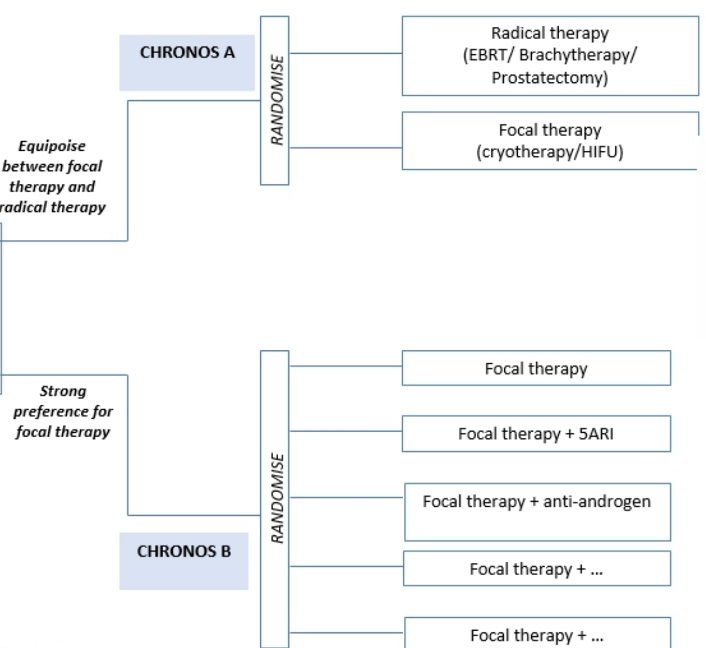
Dr. Ahmed concluded his presentation of focal cryotherapy with the following take-home messages:
- Focal prostate cryotherapy is a repeatable, effective and minimally invasive therapy
- Controlled growth of ice balls minimizes risk of damage to the neurovascular bundles, while warming catheters offer sphincteric and urethral protection
- Precise control allows you to sculpt the optimal ablation zone
- Focal cryotherapy should be the first-line treatment option for anterior prostate cancer
Presented by: Hashim U. Ahmed, PhD, FRCS(Urol), BM, BCh(Oxon), Imperial College Healthcare NHS Trust, London, UK
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, GA, USA, Twitter: @zklaassen_md, at the Virtual 2020 EAU Annual Meeting #EAU20, July 17-19, 2020.
References:
- Shah TT, Ahmed H, Kanthabalan A, et al. Focal cryotherapy of localized prostate cancer: A systematic review of the literature. Expert Rev Anticancer Ther. 2014 Nov;14(11):1337-1347.
- Shah TT, Peters M, Eldred-Evans D, et al. Early-medium-term outcomes of primary focal cryotherapy to treat nonmetastatic clinically significant prostate cancer from a prospective multicentre registry. Eur Urol 2019 Jul;76(1):98-105.


