(UroToday.com) As part of a plenary presentation at the 2020 European Association of Urology (EAU) Virtual Meeting assessing “Modern prostate cancer imaging in daily practice,” Guido Jenster, PhD, examined the role of genomics to complement imaging in patients undergoing investigation for prostate cancer.
As with any approach in medicine, there are limits to the use of imaging in prostate cancer diagnosis. Beyond issues related to the human interaction with these modalities, imaging limitations relate to both technical and non-technical factors and include the detection limits of imaging modalities, the specificity for cancerous vs non-cancerous prostate tissue, false-negative results, false-positive results, heterogeneity both within and between tumors, and molecular tumor characteristics. Genomic testing offers the ability to address many of these issues.
Dr. Jenster considered the role of imaging approaches, histopathology, and genomics across a range of clinical scenarios in the natural history of prostate cancer rather than pre-diagnostic risk stratification to the monitoring of response and progression. As highlighted in the table below, various approaches have particular strengths and weaknesses so a synergistic use may provide the most appropriate information for guiding treatment decision making at any given step in the disease process.
Highlighting data from Salmasi et al., there is concordance in the GenomeDx Genomic Prostate Score (GPS) and MRI based PiRADS score suggesting that genomic changes and radiographic changes correlate.
For pre-diagnostic risk stratification, Dr. Jenster stressed the importance of testing using biofluids including urine and blood. Based on the specific assay in question, a variety of approaches may be employed including proteins, cell-free RNA, circulating tumor cells (CTCs), cell-free DNA, metabolites, extracellular vesicles, platelets, viruses, and other micro-organisms.
A number of commercially available assays have utilized urine DNA methylation for risk stratification. While each assesses different gene loci, the underlying principle is the same.
While not commercially available, assessment of extracellular micro-vesicles allows assessment of many biomarkers including miRNAs, snoRNAs, and tRNAs. However, plasma cell-free DNA offers the potential to sequence underlying genetic changes to allow for targeted treatment.
Genomic testing may also allow the identification of novel imaging targets, such as novel prostate cancer-specific membrane proteins. A number of these are under investigation, leading to clinical trials.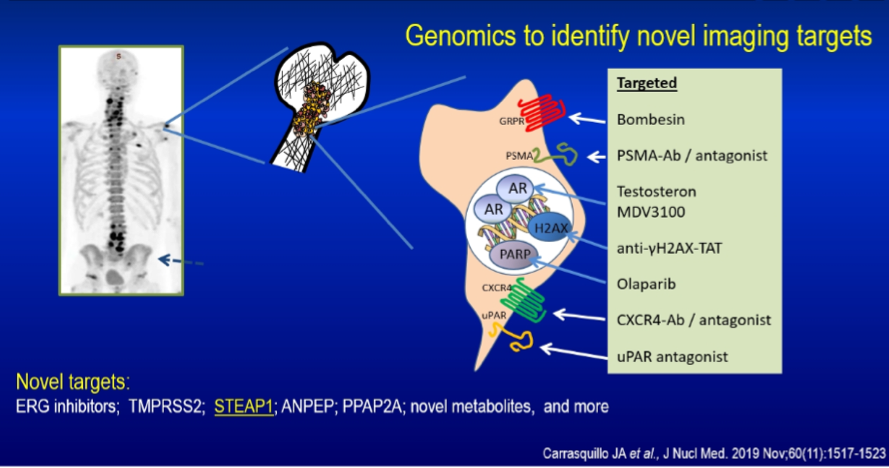
Dr. Jenster concluded highlighting the complementary nature of imaging and genomic testing to resolve within and between tumor heterogeneity, complete diagnosis and prognosis, inform personalized treatment choice, and guide further investigations.
Presented by: Guido Jenster, PhD, Associate Professor of Urology, Erasmus MC, Rotterdam, Netherlands
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Nashville, TN, USA, Twitter: @WallisCJD, at the Virtual 2020 EAU Annual Meeting #EAU20, July 17-19, 2020.


