(UroToday.com) Dr. Alison Birtle gave a talk on the ever-changing treatment paradigm of advanced prostate cancer (Figure 1). Metastatic castrate-resistant prostate cancer (mCRPC) is a lethal disease with huge heterogeneity. The same patient can have coexistence of androgen receptor-dependent and independent tumor cells. The sequencing of therapies is even more important given the use of upfront docetaxel and androgen receptor-targeted therapies (ARTA).
Figure 1 – Evolving era of prostate cancer treatment
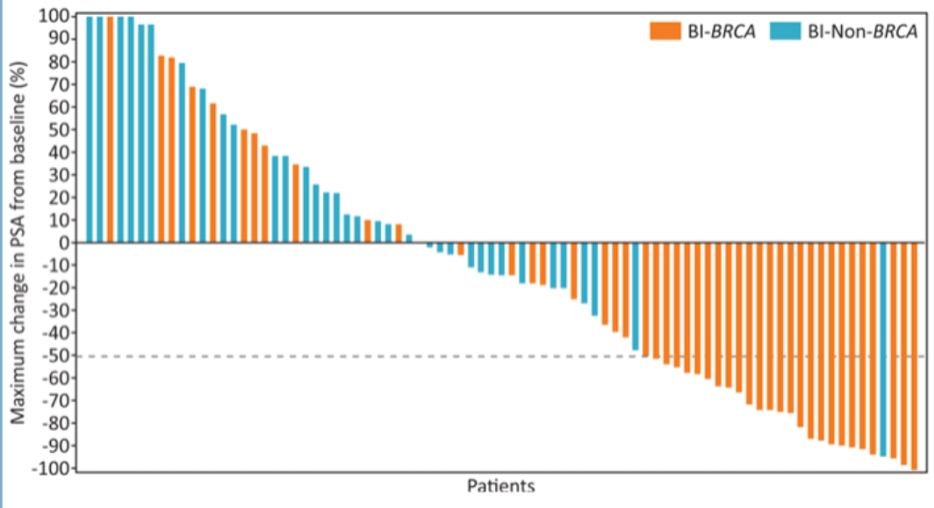
In the TRITON2 study (Figure 2), mCRPC patients with deleterious somatic or germline alteration in DNA damage repair genes, with evidence for disease progression on AR-directed therapy for prostate cancer and one prior taxane-based chemotherapy for CRPC were assessed. Patients were given rucaparib, and the objective response rate and prostate-specific antigen (PSA) response rate were assessed. Figure 3 demonstrates the best change from baseline in PSA in rucaparib-treated patients with a BRCA 1 and BRCA 2 alteration (n=96).
Figure 2 – TRITON 2 study:

Figure 3 – Best change from baseline in PSA in rucaparib-treated patients with a BRCA 1 and BRCA 2 alteration (n=96):

Next, Dr. Birtle presented the GALAHAD Trial phase 2 mCRPC biallelic DNA repair defects pre-specified study (Figure 3).
Figure 3 – Galahad trial:

The interim analyses presented at ESMO 2019 showed a significant response for bi-allelic DNA repair defects with BRCA (figure 4).
Figure 4 – GALAHAD phase 2 trial interim results ESMO 2019:
Next, Dr. Birtle discussed the NEJM paper by de Bono J. et al1 (figure 5). This study showed significant benefit in mCRPC patients treated with olaparib in radiographic progression-free survival, time to pain progression, and interim overall survival (Figure 6).
Figure 5 – Olaparib in mCRPC patients’ study:
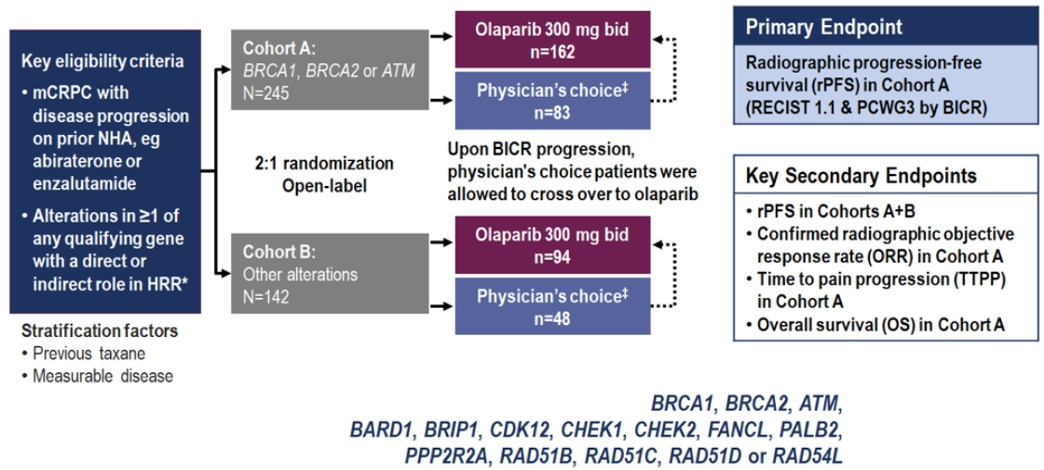
Figure 6 – Interim overall survival data in mCRPC olaparib treated patients:
Dr. Birtle moved on to discuss the TheraP (ANZUP 1603) study presented at ASCO 2020 by Dr. Michael Hofman, which was a randomized Phase 2 trial of lutetium-PSMA theranostic vs. Cabazitaxel in mCRPC patients progressing after docetaxel (Figure 7). Study endpoints included PSA response, PSA progression-free survival, and adverse events. Other endpoints included objective tumor response, pain response, radiographic progression-free survival, pain progression-free survival, overall progression-free survival, health-related quality of life, and overall survival.
Figure 7 – TheraP trial design:

This study demonstrated that Lu-PSMA was associated with a 29% absolute greater PSA response rate of more than 50% compared to cabazitaxel (figure 8).
The strengths of this study include the fact that this was the first randomized trial assessing Lu-PSMA. The study had an active and clinically relevant control arm. The authors used F-FDG, and Ga-PSMA PET/CT scans to select patients. Lastly, there was a large difference in the primary endpoint.
The limitations of this study include the fact that we need to wait for further follow up for results of other key secondary endpoints, including radiographic progression-free survival, quality of life, progression-free survival, and overall survival. Moreover, this was not a blinded study.
Importantly, the adverse events associated with Lu-PSMA were lower when compared to those associated with cabazitaxel. This was true for both grade 1 & 2 adverse events and grade 3 & 4 adverse events.
Figure 8 – TheraP trial primary endpoint (PSA >=50% response):
Dr. Birtle moved on and provided some summary points and key take-home messages. Although Lu-PSMA led to a far superior PSA response rate compared to cabazitaxel, this is still not a practice-changing study, as PSA 50 is not a true surrogate for overall survival. However, these data do support increased enthusiasm for the promise of Lu-PSMA. Researchers eagerly await the results of the VISION study comparing the standard of care plus Lu-PSMA vs. SOC only.
Next, Dr. Birtle discussed the KEYNOTE 199 study assessing pembrolizumab plus enzalutamide for enzalutamide- resistant mCRPC (Figure 9). The study demonstrated the added benefit of pembrolizumab to enzalutamide after enzalutamide resistance. The results showed an objective response rate of 12%. Overall survival was not reached, and the incidents of all grade three rash resolved with standard of care treatment.
Figure 9 -KEYNOTE 199 study:
Lastly, Dr. Birtle discussed the recently published CARD study2 (figure 10), demonstrating a clear benefit to cabazitaxel in imaging-based progression-free survival (Figure 11), and overall survival (figure 12).
Figure 11 – The CARD study:

Figure 12– CARD study imaging-based progression-free survival:
Dr. Birtle concluded her talk stating that we are getting close to an ideal treatment sequence, and precision medicine is here to stay.
Presented by: Alison Birtle, MB, BS, MRCP, FRCR, MD, Lancashire Teaching Hospitals, NHS, United Kingdom
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, @GoldbergHanan at the 35th Annual EAU Congress, 2020 Virtual Program #EAU20, July 17-19, 2020.
References:
1. de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. New England Journal of Medicine 2020; 382(22): 2091-102.
2. de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. New England Journal of Medicine 2019; 381(26): 2506-18.


