Patients with localized prostate cancer, treated by radical prostatectomy (R0 or R1), with a PSA-level post-radical prostatectomy ≥0.2 ng/mL and ≤2 ng/mL at randomization and N0 M0 on imaging were included. Patients were randomized (1:1) to radiotherapy alone or 6 months of degarelix hormone therapy plus radiotherapy. The trial schema is as follows: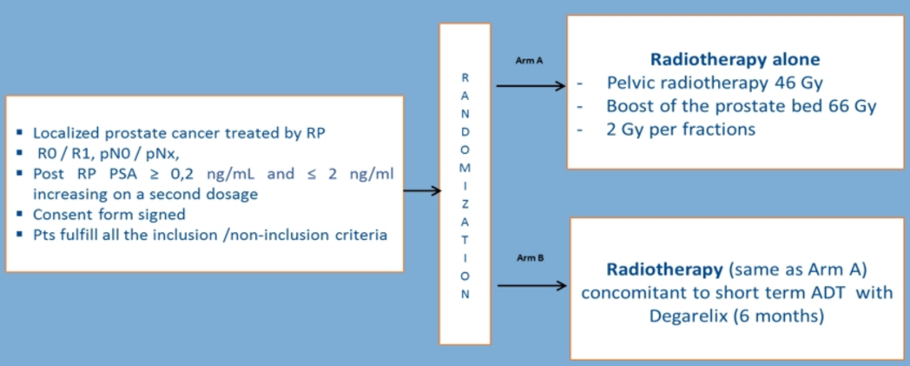
Radiotherapy consisted of pelvic irradiation (46 Gy in 23 fractions) with a boost on the prostate bed (66 Gy in 33 fractions). The primary endpoint was event-free survival, and acute and late toxicities were evaluated as secondary endpoints and scored using CTCAE v4.0. Quality of life was assessed with QLQ-C30 and QLQ-PR25 questionnaires at 12 and 24 months. Late toxicity was reported at 24 months.
From January 2013 to September 2015, 125 patients were included (radiotherapy arm: 64 patients; hormone therapy plus radiotherapy arm: 61 patients). Median follow up was 38.1 months (95% CI 31.4-44.1), and the baseline characteristics were well-balanced between both arms: median age was 66 years (50-77), 92% of patients had a performance status of ECOG 0, median Gleason score was 7 (range 3-9), median PSA level was 0.3 ng/mL (range 0.09-1.82) post-radical prostatectomy and 0.6 ng/mL (range 0.12-3.65) at randomization. All patients received 33 fractions of radiotherapy. In the hormone therapy with radiotherapy arm, 98.4% of patients received the 6 months of hormone therapy planned. At 24 months, there was no difference in late genitourinary or gastrointestinal toxicity observed between both arms (p=0.145). Grade 3 late toxicities were reported for 15/125 patients (12%): 8/64 (6.5%) in the radiotherapy arm and 7/61 (5.5%) in the hormone therapy with radiotherapy arm (not significant); no toxicity grade >3 was observed. Quality of life was evaluated at 12 months of follow up in 80% of patients in the radiotherapy arm, and 89% in hormone therapy with radiotherapy arm, and at 24 months for 59% and 77% of patients, respectively. At 12 months, hormone therapy-related symptoms were more important in hormone therapy with radiotherapy arm (p=0.04). At 24 months, no difference in QLQC-30 or QLQ-PR25 analysis was reported.
Dr. Sargos concluded his presentation of GETUG-AFU 22 with the following remarks:
- This is the only randomized phase II trial comparing radiotherapy versus hormone therapy with radiotherapy in a very selected postoperative population (median pre-radiotherapy PSA was 0.6 ng/mL)
- Late toxicity by physicians was low and no difference was noted between the two arms at two years for genitourinary/gastrointestinal or maximal toxicity
- Quality of life assessed with questionnaires showed an impact of ADT related symptoms at one year, but there was no difference with longer follow-up
- Final results for efficacy are still pending and should be available for next year
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the Virtual 2020 EAU Annual Meeting #EAU20, July 17-19, 2020


