Dr. Keisuke Shigeta from Tokyo, Japan joined the Joint Session of the European Association of Urology and Japanese Urological Association at the 2021 EAU annual meeting to discuss treatment challenges in BCG unresponsive non-muscle invasive bladder cancer (NMIBC). Dr. Shigeta notes that there are important definitions for the BCG treatment of NMIBC, highlighted as follows:
- BCG refractory: (i) T1/G3 or high-grade NMIBC present at 3 months, (ii) TaG3/HG or CIS present at 3 or 6 months after BCG (after the 2nd induction or first maintenance dose), (iii) high-grade tumor appearing during BCG therapy
- BCG relapsing: recurrence of high-grade tumor after BCG maintenance
- BCG intolerance: BCG abortion with severe side effects
- BCG unresponsive: BCG refractory + TaT1/high-grade within 6 months, or CIS within 12 months after completion of BCG exposure
The EAU 2021 guidelines highly recommend radical cystectomy for BCG unresponsive bladder cancer, whereas bladder preserving strategies are recommended for patients unsuitable for radical cystectomy. Furthermore, the 2020 NCCN guidelines suggest that recurrent or persistent disease which is cTa, T1, or Tis should proceed with radical cystectomy, whereas other options include pembrolizumab or other intravesical agents for those that do not desire cystectomy. Historical data from 2006-2012 suggests that the progression rates for BCG failure progression patients ranges from 31-47%, as highlighted in the following table:
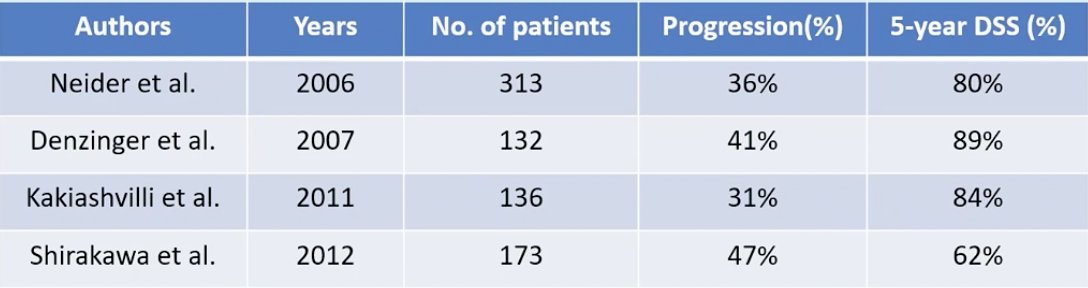
Dr. Shigeta emphasized that in addition to radical cystectomy being the preferred option for patients with BCG unresponsive disease, there are several other benefits:
- Radical cystectomy provides the greatest opportunity for cure (DSS rates of 80-90%)
- It covers those patients upstaged on transurethral resection (upstaging at the time of cystectomy for T1HG tumors ranges from 25-50%)
- Radical cystectomy enables lymphadenectomy, which improves staging and may confer a therapeutic benefit (5-20% of patients may harbor occult lymph node metastases at the time of radical cystectomy)
- Radical cystectomy obviates the need for further intravesical therapy and thus simplifies the follow-up regimen
Looking at unpublished data from Dr. Shigeta’s institution, he notes that among 987 NMIBC patients, 109 (11.0%) were classified as BCG unresponsive. These patients had a median age of 70 years (range 40-86), with median follow-up of 49.8 months (range: 7.6-223.1). Ultimately, 34.9% of these patients progressed to muscle invasive disease; overall 67% of BCG unresponsive patients underwent radical cystectomy and 33% chose bladder preservation strategies. The 5-year recurrence free survival rate was 69.3%; the 5-year cancer specific survival rate was 80.3% for patients in the radical cystectomy group and 60.4% in the bladder preservation group (p=0.004):
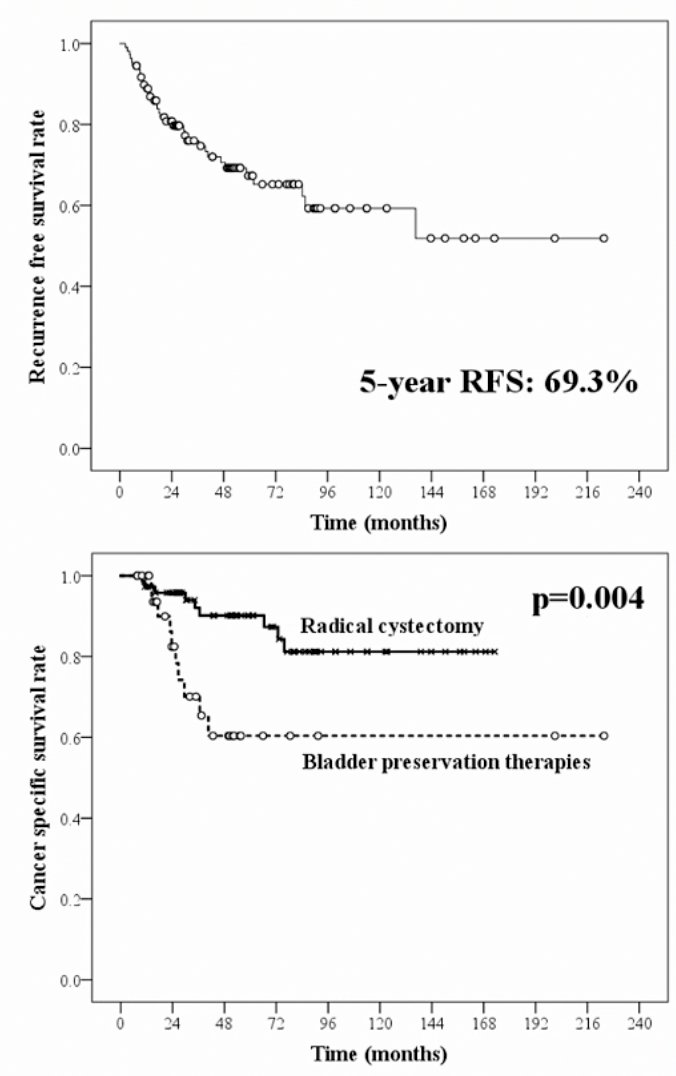
Recent data suggest that guideline adherence for radical cystectomy significantly affects survival outcomes in non-muscle-invasive bladder cancer. Dr. Shigeta’s group assessed 267 cTa-4N0-2M0 bladder cancer patients, 70 of which underwent radical cystectomy that progressed from non-muscle-invasive bladder cancer.1 Patients who followed the guidelines from initial transurethral resection of bladder tumors to radical cystectomy were defined as the guideline adherent group (n = 52), while those who did not were the guideline non-adherent group (n = 18). Five-year recurrence-free survival and cancer-specific survival rates for the guideline non-adherent group vs guideline adherent group were 38.9% versus 69.8% (p = 0.018) and 52.7% versus 80.1% (p = 0.006), respectively. Furthermore, on multivariable analysis, guideline non-adherence was an independent predictor for disease recurrence (HR 2.81, p = 0.008) and cancer-specific death (HR 4.04, p = 0.003).
Dr. Shigeta also briefly highlighted other bladder preservation therapies for BCG unresponsive disease, including:
- Intravesical chemoimmunotherapy (ie. 8 mg Mycobacterium phlei cell wall-nucleic acid complex – 1 year RFS 30%)
- Intravesical chemotherapy (ie. gemcitabine, Mitomycin C, epirubicin)
- Device assisted therapy (ie. electromotive drug administration of Mitomycin C)
Dr. Shigeta noted that several clinical trials of PD-1/PD-L1 are ongoing and have reported initial data, including the SWOG S1605 trial of atezolizumab q3 weeks x 1 year, and the KEYNOTE-057 trial of pembrolizumab q3 weeks x 24 months, recently published in Lancet Oncology.2
Dr. Shigeta concluded his presentation of treatment challenges to BCG unresponsive NMIBC by highlighting additional phase I/II ongoing clinical trials, as well as the following summary statements:
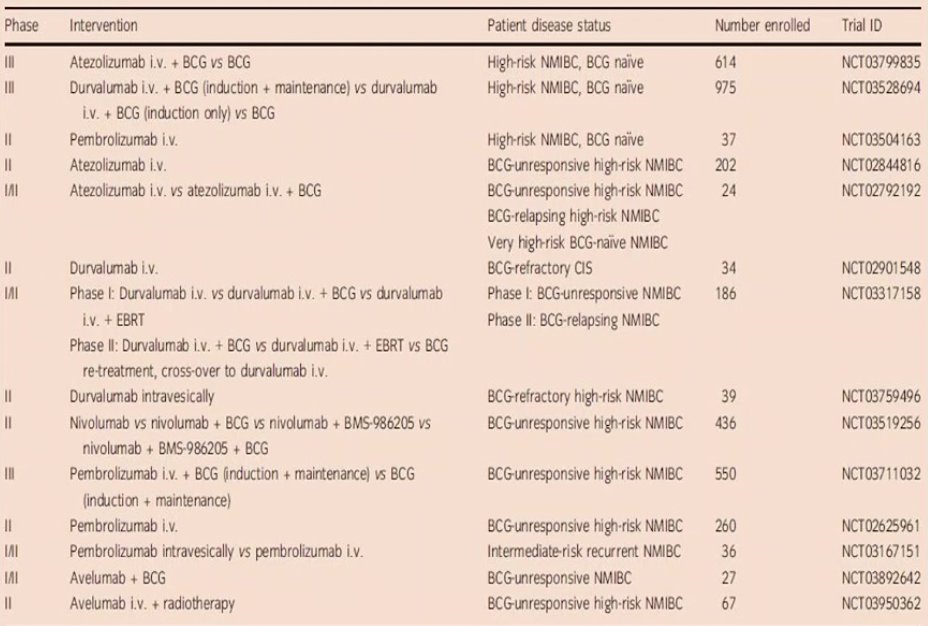
- BCG unresponsive NMIBC is a new subclassification of BCG failure, which includes BCG refractory + BCG relapsing patients with aggressive tumor features
- Although BCG unresponsive tumors make up only 10-20% of NMIBC patients, their subsequent management comprises an unmet clinical need
- Radical cystectomy is the only promising treatment option for cure in BCG unresponsive NMIBC patients, however clinical trials including intravesical PD-1/PD-L1 blockade are ongoing to provide bladder preserving options for these patients
Presented by: Keisuke Shigeta, MD, Department of Urology, Keio University School of Medicine, Tokyo, Japan
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
- Kayama E, Shigeta K, Kikuchi E, et al. Guideline adherence for radical cystectomy significantly affects survival outcomes in non-muscle-invasive bladder cancer patients. Jpn J Clin Oncol. 2021 May 3 [Epub ahead of print].
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021 May 26;S1470-2045(21)00147-9.


