- Second-line treatment for metastatic disease: KEYNOTE-045,1 IMvigor 2112
- First-line treatment of metastatic disease: KEYNOTE-052,3 IMvigor 2104 in patients whose tumor expresses PD-L1
- First-line maintenance treatment: JAVELIN Bladder 1005
Given the above success of immunotherapy in bladder cancer, there is great interest in the possibility of moving immunotherapy into the neoadjuvant disease space. Currently, there are many ongoing clinical trials for neoadjuvant immunotherapy:6
Two clinical trials have assessed single-agent immunotherapy to date, including the PURE-017 and ABACUS8 phase II trials. PURE-01 enrolled 143 patients that were treated with 3 cycles of pembrolizumab, resulting in a pT0 rate of 37%. ABACUS enrolled 95 patients that were treated with two cycles of atezolizumab, resulting in a pT0 rate of 31%. Three clinical trials have assessed the utility of immunotherapy combination therapy, including the NABUCCO (ipilimumab + nivolumab x 3), DUTRENEO (durvalumab + tremelimumab x 3), and durvalumab + tremelimumab x 2 (NCT02812420). As follows is a summary of these trials:
Additionally, five trials have assessed immunotherapy in combination with chemotherapy including the BLASST-1 trial (Gem-Cis + nivolumab x 4), the SAKK06/17 trial (Gem-Cis + durvalumab x 4), the GU14-188 Cohort 1 trial (Gem-Cis + pembrolizumab x 5), the GU14-188 Cohort 2 trial (Gem x 3 + pembrolizumab x 5), and the Gem-Cis x 4 + atezolizumab x 2 (NCT02989584) trial. A summary of the outcomes of these trials is as follows: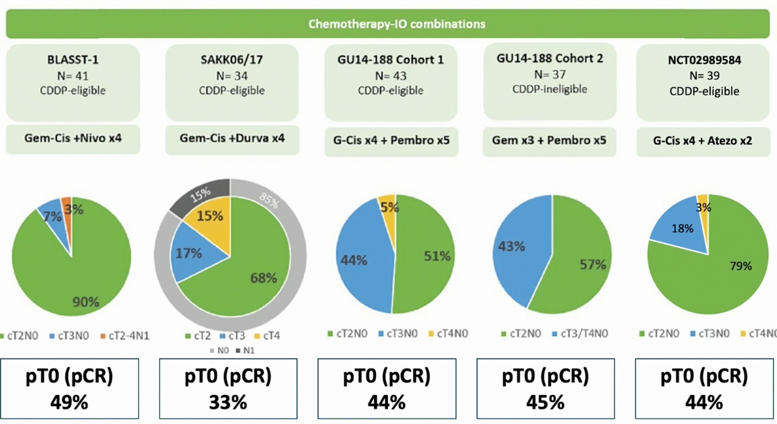
To date, only one trial has assessed immunotherapy plus targeted agents, which was the NEODURVARIB trial (durvalumab x 2 + olaparib x 6 weeks), reporting a pT0 rate of 50% among 29 treated patients. The safety of these neoadjuvant trials has been satisfactory with treatment-related adverse events >= grade 3 ranging from 8%-55% and surgical complications >= grade 3 ranging from 17%-27%:

Dr. Audenet concluded his presentation with the following summary points:
- Immune checkpoint inhibitor therapy before radical cystectomy for MIBC shows good antitumor efficacy, with pathologic complete response rates comparable to historic neoadjuvant chemotherapy trials. Additionally, these treatments were well tolerated with radical cystectomy generally proceeding without delay
- However, there are many questions that remain to be answered:
- What is the long-term survival data?
- What is the efficacy of neoadjuvant immunotherapy as compared to neoadjuvant chemotherapy?
- What is the optimal schedule and timing between neoadjuvant immunotherapy and surgery?
- How do we identify patients most likely to benefit from immunotherapy?
Presented by: Francois Audenet, MD, Urologist, Hopital Europeen Georges Pompidou, AP-HP, Centre-Universite de Paris, Paris, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376(11):1015-1026.
- Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomized controlled trial. Lancet 2018;391:748-757.
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18(11):1483-1492.
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016;387(10031):1909-1920.
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.
- Rouanne M, Bajorin DF, Hannan R, et al. Rationale and outcomes for neoadjuvant immunotherapy in urothelial carcinoma of the bladder. Eur Urol Oncol. 2020 Dec;3(6):728-738.
- Necchi A, Raggi D, Gallina A, et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur Urol 2020 Apr;77(4):439-446.
- Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019 Nov;25(11):1706-1714.


