The DANUBE trial was previously presented at ESMO 2020. At this time, atezolizumab and pembrolizumab are licensed in cisplatin-ineligible, PD-L1-positive mUC patients based on single arm trials. Randomized phase 3 trials have not yet shown OS benefit for these agents over chemotherapy.
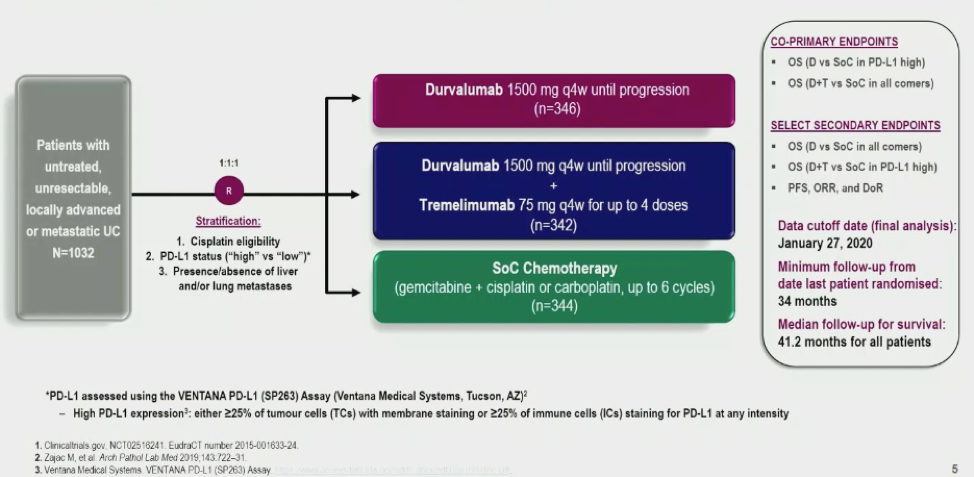
The trial design of DANUBE is shown below:
Ultimately, as seen below, the study did not meet its primary endpoint.

There was no significant benefit to Durva or Durva/Treme in median OS compared to SOC chemotherapy (gem/cis or gem/carbo, at discretion of treating physician).
In the first part of this exploratory analysis, Dr. Powles first compares the outcomes of the gem/cis to gem/carbo patients in the SOC control arm. This is to provide a more modern comparison of these regimens in patients who had good performance status (as PS 2 patients were excluded for the DANUBE trial).
Ultimately, the main difference between the two groups of patients was kidney function – limiting cisplatin eligibility.
With regards to PFS – no significant difference was noted (median PFS 5.7 months for carboplatin vs. 7.0 month for cisplatin, p = 0.31).
With regards to OS, as seen below, median OS was better with cisplatin (14.2 months vs. 10.6 months, p = 0.0889). While not statistically significant, this was not the primary endpoint of the study.
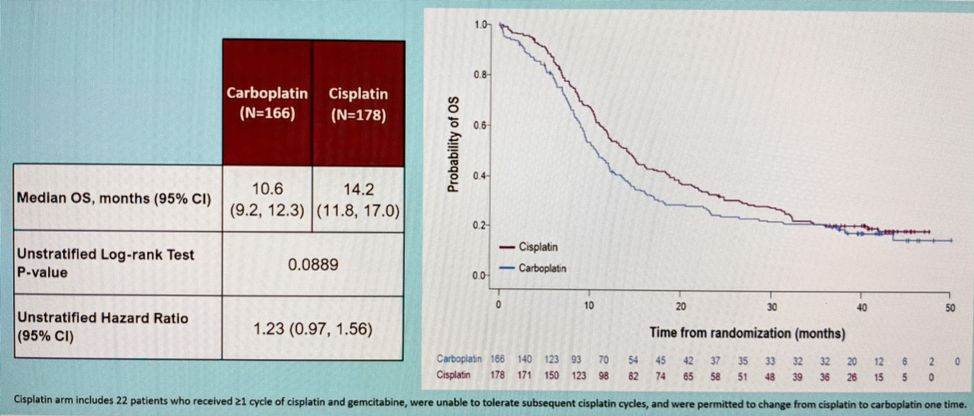
This demonstrates that carboplatin patients do better than historical studies suggested in patients with good performance status.
Next, he went into the exploratory analysis of chemotherapy vs immunotherapy.
In the ITT population, when comparing Durva to SOC – there was no significant difference, regardless of cisplatin eligibility.
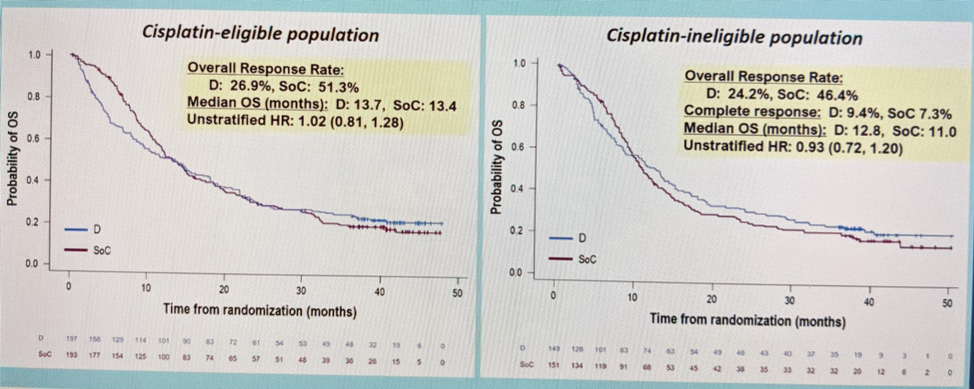
When looking at Durva/Treme vs. SOC, there is no significant in the cisplatin-eligible population. But, in the cisplatin-ineligible population, there appears to be a hint of improvement – but not statistically significant.
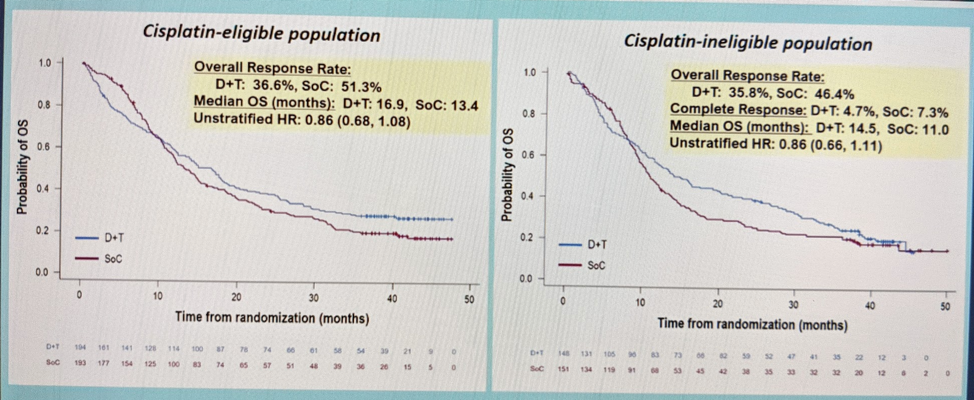
He then broke it down based on PD-L1 status – but even in the PD-L1 high cohort, there was no difference between Durva vs. SOC. But, in the cisplatin-ineligible cohort with PD-L1 high, response rates were similar to other ICI therapies (HR 0.87).
Utilizing different PD-L1 algorithms, there was similar overall survival HR benefit, regardless of PD-L1 algorithm utilized. Of note, patients with IC >= 5%, longer survival was observed, irrespective of treatment.
He then also looked at differential outcomes based on blood tumor mutational burden (TMB) and tissue TMB – and did note that OS favored Durva/Treme over SOC at prespecificied bTMB cutoff >= 24 mut/Mb, tTMB cutoff >= 10 mut/Mb.
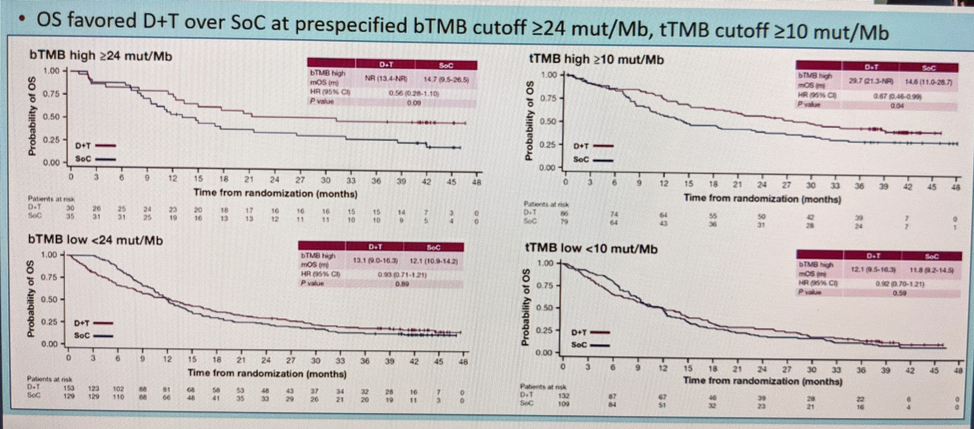
Based on all of this, he concluded the following:
- Carbo/gem patient have outcomes which appeared longer than historical controls, with response rates and PFS in line with cisplatin based chemotherapy
- Durvalumab showed activity in the cis-ineligible, PD-L1 high population – in line with results for other ICIs in this population
- Treme appeared to improve the activity of Durva when given in combination
- Biomarker analysis suggests components of immune and tumor expression may be relevant in determining outcomes
- Randomized Phase 3 NILE and VOLGA trials further explore the Durva/Treme combo
Dr. De Santis then followed this with her discussion, in which she raised some important points.
With regards to the issue of modern cis vs. carbo comparison, she agrees that carbo/gem is more effective than in historical controls. Which is why guidelines now talk about platinum ineligibility – not just cisplatinum-ineligibility.

Looking at carbo/gem outcomes across other trials:

The outcomes in DANUBE are in line with the other trials – it should be noted that other studies did include PS2 patients, so this might account for some of the differences in OS outcomes.
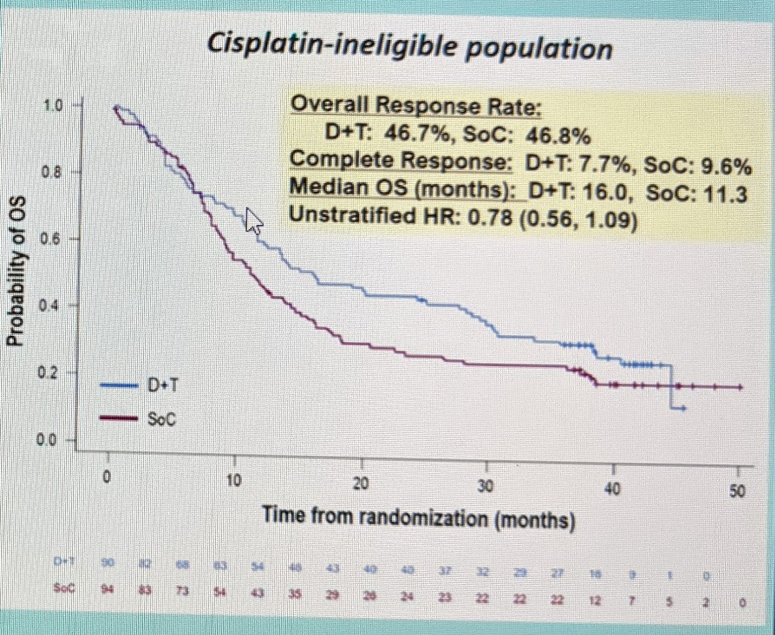
She next addressed the comparison of the Durva/Treme combination therapy against SOC therapy. She noted the best response relative to SOC appeared to be for the combination in cisplatin-ineligible patients with PD-L1 high status:
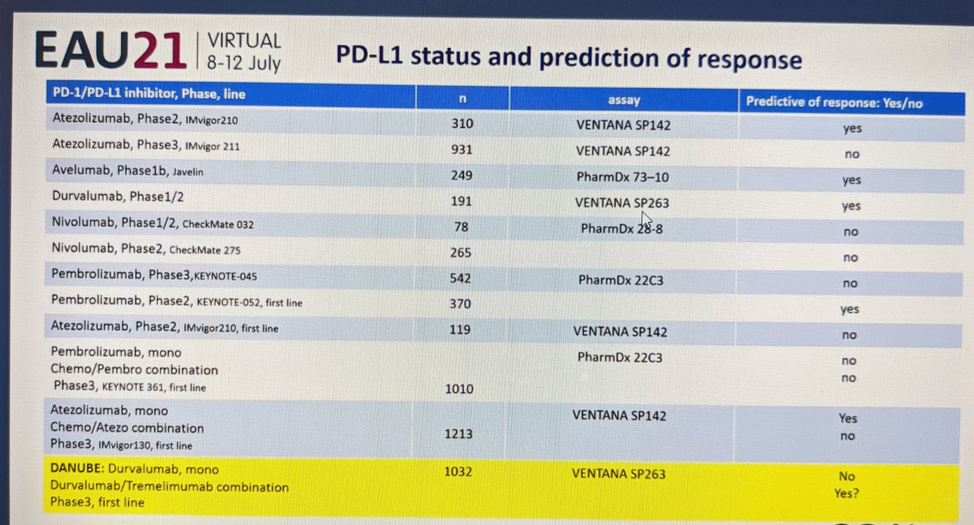
She highlighted the technical challenges of using PD-L1 as a biomarker. As seen in these multiple studies, the use of it as a biomarker of response has been mixed:
She also briefly touched on high tumor mutational burden (TMB) as a potential marker of IO response. But, she did note that its utility as a biomarker has not been clearly established. She noted in the exploratory analysis of the DANUBE study that patients with high blood TMB and PD-L1 high had the best response to combination therapy:
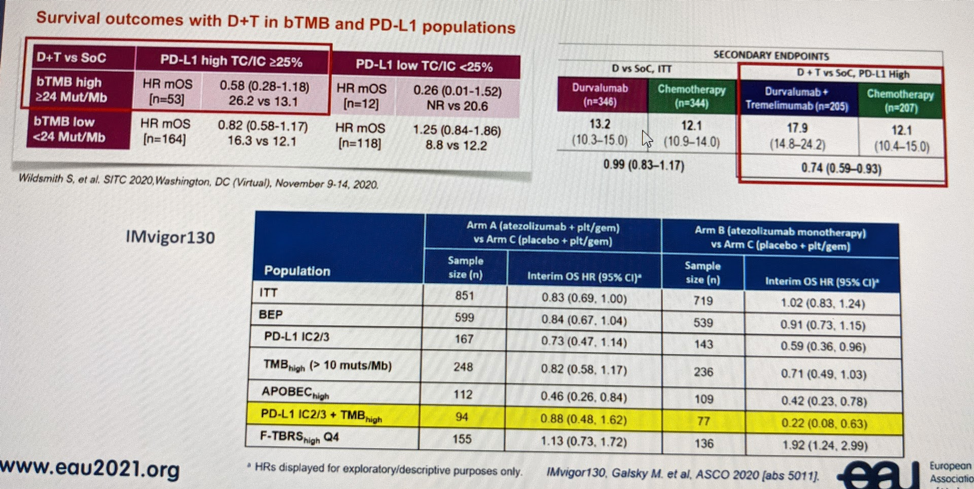
This is similar to the results seen in the IMvigor130 study (ASCO 2020 abstract), the table in the bottom of the picture.
Based on all of the above, she concludes the following:
Carboplatin remains a valid treatment option cisplatin ineligible patients
- better performance in contemporary PS0-1 patients than in historical cis-unfit populations
Conflicting data on PD-L1 positivity and predictive potential for ICI monotherapy
Addition of TMB to PD-L1 as a combined biomarker for ICI treatment selection holds some promise, but needs more data and validation.
Presented by: Thomas Powles, MBBS, MRCP, MD, Barts Cancer Institute, London, United Kingdom
DISCUSSANT: Maria De Santis, MD, Charité Medical University Hospital, Berlin, Germany
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Assistant Professor of Urology, Sidney Kimmel Cancer Center, Thomas Jefferson University, @tchandra_uromd on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
- Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, Ogawa O, Park SH, Lee JL, De Giorgi U, Bögemann M, Bamias A, Eigl BJ, Gurney H, Mukherjee SD, Fradet Y, Skoneczna I, Tsiatas M, Novikov A, Suárez C, Fay AP, Duran I, Necchi A, Wildsmith S, He P, Angra N, Gupta AK, Levin W, Bellmunt J; DANUBE study investigators. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020 Dec;21(12):1574-1588. doi: 10.1016/S1470-2045(20)30541-6. Epub 2020 Sep 21. Erratum in: Lancet Oncol. 2021 Jan;22(1):e5. PMID: 32971005.


