First and foremost, he makes it very clear that the PD-L1 biomarker is faulty at best. Unfortunately, the “PD-L1 biomarker” consists of many different biomarkers and should be considered as such. Each PD-L1 biomarker was developed separately and each test run in different settings will yield different results. Each also used different cutoffs ranging from 1-25%. Moreover, as he notes, in most of the randomized controlled trials (RCTs), the biomarker was not predictive of response anyway – there was response irrespective of the PD-L1 status.

In the JAVELIN Bladder 100 study,1 which was positive in the intention-to-treat (ITT) cohort, neither PD-L1 in the tumor cell (TC) or immune cell (IC) alone fully predicted overall survival (OS) benefit – but when broken down, it did look like the TC staining was more predictive than IC (HR 0.35 vs. HR 0.61).
In the DANUBE trial analysis of biomarkers,2 which he presented earlier today when looking at OS stratified by TC/IC PD-L1 status, similar OS benefit was noted regardless of algorithm. Of note, with IC PD-L1 >=5%, longer survival was noted, irrespective of treatment.
While PD-L1 as a biomarker is limited to the PD-1/PD-L1 status, and in his mind, probably not going to be useful in the future, tumor mutational burden (TMB) is a different story. In JAVELIN Bladder 100, TMB was an independent predictor of better response to avelumab:

But TMB nor PD-L1 status alone fully predicted OS benefit.
His main takeaway from TMB status is that increased TMB yields more antigens, which likely results in increased response to all checkpoint inhibitors, not just PD-L1 or PD-1 inhibitors.
In the same study, they also noted that multiple immune cell signatures (T-cell, NK cell, Macrophage, dendritic cell, and B-cell) were associated with improved OS response to avelumab. Non-immunogenic signaling pathways (Notch, Hedgehog, TGFb, Angiogenesis) were also inversely associated with OS response.
This all points to a much more complicated pathway that we previously acknowledged.
In the BISCAY trial, the authors attempted to take an adaptive, biomarker-driven pathway to select durvalumab combination therapy for metastatic urothelial carcinoma. This was previously presented at ESMO 2019.
The study design is below:

Ultimately this study was negative, indicating how difficult biomarker selection can be.
He next briefly touched upon ctDNA as a biomarker. Dr. Schwend had previously presented this data on the ctDNA data from IMvigor010,4 specifically the analysis stratified by ctDNA status. In exploratory survival analysis, ctDNA status was strongly predictive of response to adjuvant atezolizumab.
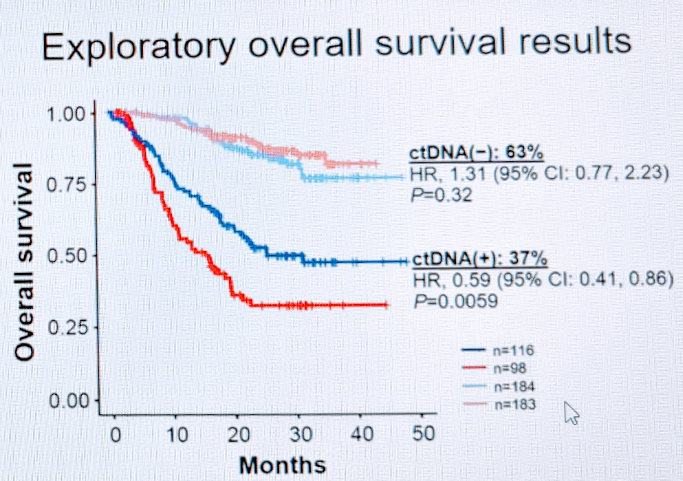
So, in summary, he concludes:
1. PD-L1 and TMB have not yet helped patients and may never do so for UC.
2. Innate and adaptive immunity are relevant
3. New approaches, including circulating biomarkers, TLS, and multiple biomarkers may be needed.
Presented by: Thomas Powles, MD, MBBS, MRCP, Professor of Genitourinary Oncology, Director, Barts Cancer Centre, Lead for Solid Tumour Research London, UK
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Assistant Professor of Urology, Sidney Kimmel Cancer Center, Thomas Jefferson University, @tchandra_uromd on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
1. Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020 Sep 24;383(13):1218-1230. doi: 10.1056/NEJMoa2002788. Epub 2020 Sep 18. PMID: 32945632.
2. Powles T, van der Heijden MS, Castellano D, et al.; DANUBE study investigators. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced, or metastatic urothelial carcinoma (DANUBE): a randomized, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020 Dec;21(12):1574-1588. doi: 10.1016/S1470-2045(20)30541-6. Epub 2020 Sep 21. Erratum in: Lancet Oncol. 2021 Jan;22(1):e5. PMID: 32971005.
3. Powles T, Carroll D, Chowdhury S, et al. An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat Med. 2021 May;27(5):793-801. doi: 10.1038/s41591-0
4. Bellmunt J, Hussain M, Gschwend JE, et al.; IMvigor010 Study Group. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2021 Apr;22(4):525-537. doi: 10.1016/S1470-2045(21)00004-8. Epub 2021 Mar 12. PMID: 33721560.


