(UroToday.com) The joint session of the European Association of Urology (EAU) and the Canadian Urological Association (CUA) included a presentation by Dr. Antonio Finelli discussing the importance of making indications for prostate biopsy as precise as possible. Dr. Finelli notes that the question of who and how we should biopsy men is a difficult question to address. Looking at the Canadian guidelines, screening is broadly based on PSA level with adjunctive testing and prostate biopsy considered in those with an elevated PSA >3 ng/ml, based on the threshold of 3 ng/mL in the ERSPC trial and 2.5-3.4 ng/mL in the Goteborg trial. As follows is the algorithm from the CUA guidelines:
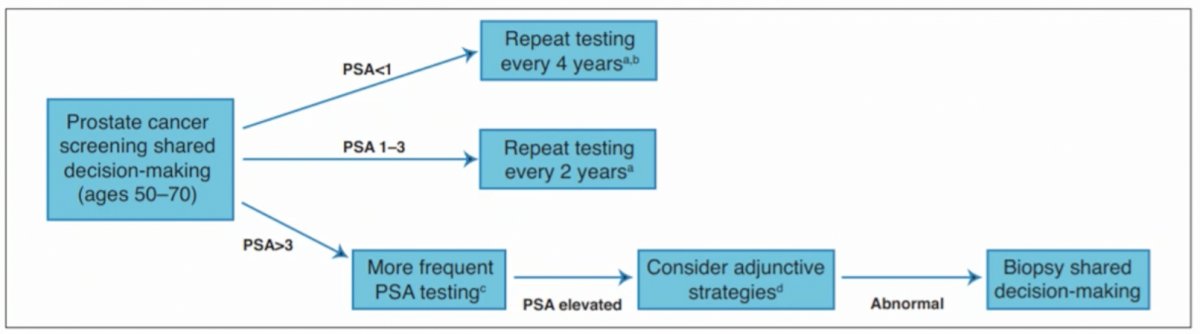
Similar to the CUA guidelines, the EAU recommends adjunctive testing (ie. risk assessment) as the key to best selecting patients, including risk calculators, imaging (MRI +/- ultrasound), and biomarkers (4Kscore, SelectMDx, PHI, PCA3, etc). While all of these are feasible, the most robust evidence points towards utilization of imaging and risk calculators.
Dr. Finelli highlighted that the new 2021 Cancer Care Ontario guidelines now also recommend pre-biopsy MRI in biopsy naïve patients with combination targeted and systematic biopsy. Positive MRIs are delineated as PI-RADS 4 or 5 and an equivocal MRI is associated with a PI-RADS 3 lesion (recommend biopsy or follow-up). Over the last 5 years, we have accumulated evidence to suggest that MRI and targeted biopsies are here to stay. PROMIS1 demonstrated that MRI outperformed systematic biopsy with increased sensitivity (93%) and NPV (89%), with the possibility for biopsy avoidance in 27% of cases (negative MRI). PRECISION2 demonstrated an increased detection of clinically significant prostate cancer (38% vs 26%), decreased insignificant prostate cancer (9% vs 22%), and biopsy avoidance in 28%. A Cochrane review similarly concluded that an MRI pathway detected 5% more clinically significant cancers and 1/3 fewer overdiagnoses of clinically insignificant cancers.
PRECISE3 is the newest Canadian, multicenter randomized controlled trial at five centers including biopsy naïve men with a clinical suspicion of prostate cancer defined as a >=5% chance of GG2 or higher using the PCa Prevention Trial Risk Calculator. There were 83/221 men (37%) that had a negative MRI result and avoided biopsy; GG2 and higher cancers were identified in 67/225 men (30%) who underwent TRUS biopsy versus 79/227 (35%) allocated to MRI (with an absolute difference of +5%). Furthermore, GG1 cancers were reduced by more than half in the MRI arm (22% to 10%; risk difference, -11.6%). Dr. Finelli emphasized that regardless of threshold, combination biopsy (targeted + systematic) offers the greatest yield with different cancers being detected by different methods.
Dr. Finelli then switched gears and discussed micro-ultrasound for the detection of prostate cancer. Published earlier this year, Klotz et al.4 assessed the sensitivity, specificity, negative predictive value, and positive predictive value of mpMRI with high-resolution micro-ultrasound imaging for the detection of clinically significant prostate cancer among 1,040 patients across 11 sites in a prospective registry. Among these patients, 39.5% had clinically significant prostate cancer. Micro-ultrasound and mpMRI sensitivity was 94% vs. 90%, respectively (p=0.03), and negative predictive value was 85% vs. 77%, respectively. Specificities of micro-ultrasound and MRI were both 22%, with similar positive predictive value (44% vs. 43%). Taken together, micro-ultrasound offers a potentially lower-cost alternative to MRI.
There are several validated and available risk calculators for assessing prostate cancer risk prior to biopsy. The ERSPC RC3 has one of the highest discriminative value (AUC = 0.79) for prostate cancer, and the PCPT risk calculator also performed well in predicting clinically significant prostate cancer (AUC = 0.71). Importantly, risk calculators can be used as a stand-alone tool (to be combined with patient-specific information and preferences) or in combination with imaging. There are several biomarkers on the market (ie. PHI, 4Kscore) for assessing prostate cancer risk, of which a sensitive biomarker may help rule out prostate cancer risk in some patients in a non-invasive manner, but often at a considerable out-of-pocket cost.
Dr. Finelli concluded his presentation with the following final thoughts with regards to risk assessment:
- No test is perfect, whether we are discussing MRI, ultrasound, biomarkers, or risk calculators
- Even within each modality, different thresholds have different levels of diagnostic performance
- While each tool can potentially better identify patients who are at risk, the large trade-offs between sensitivity and specificity mean that many men may still need a biopsy, while others may harbor missed cancers
- Ultimately, the takeaway from all of these additional tests and indicators is the shared decision-making process. The threshold for biopsy varies for each man and the biopsy decision must incorporate patient preferences and personal risk tolerance
- The tools available to us inform the decision, but they do not make the decision
Presented by: Antonio Finelli, MD, University Hospital Network, Toronto, Ontario, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017;389(10071):815-822.
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med 2018;378(19):1767-1777.
- Klotz L, Chin J, Black PC, et al. Comparison of Multiparametric Magnetic Resonance Imaging-Targeted Biopsy with Systematic Transrectal Ultrasonography Biopsy for Biopsy-Naïve Men at Risk of Prostate Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021 Apr 1;7(4):534-542.
- Klotz L, Lughezzani G, Maffei D, et al. Comparison of micro-ultrasound and multiparametric magnetic resonance imaging for prostate cancer: A multicenter, prospective analysis. Can Urol Assoc J. 2021 Jan;15(1):E11-E16.


