Dr. Murphy started his presentation noting that conventional navigation would be akin to using the London A to Z atlas to map a route across London, whereas the novel navigation is akin to using Google maps on your smartphone to direct your journey. Similarly, bone scan and CT scan for staging are traditionally referred to as conventional imaging, while PSMA PET/CT is the latest novel imaging modality in advanced prostate cancer diagnostics. Dr. Murphy notes that PSMA PET/CT has established itself for its accuracy in regional disease, distant disease, and de novo and recurrent disease.
Dr. Murphy then proceeded to discuss the seminal proPSMA prospective, randomized, multi-center clinical trial [1]. To be eligible for inclusion in proPSMA, men must have had at least one high-risk factor including PSA >= 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. Patients who had undergoing staging investigations (apart from prostate MRI) within eight weeks prior to randomization were excluded. Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging performed using bone scan and CT or PSMA PET/CT. Patients who were randomized to conventional imaging underwent an abdominopelvic CT scan with contrast as well as a technetium-99m bone scan with SPECT CT of chest, abdomen, and pelvic in keeping with the standard of care. For patients randomized to PET/CT, gallium-68 PSMA-11 PET/CT was performed. In patients who had fewer than three unequivocal sites of metastasis, cross-over imaging for confirmation was performed within 14 days. Confirmatory testing following imaging was performed at the discretion of the treating physician and included biopsy confirmation.
Between 2017 and 2018, the trial randomly assigned 302 patients of whom 300 received assigned first-line imaging. In the primary outcome assessment, PSMA PET-CT had a 27% absolute greater AUC for accuracy compared to conventional imaging (95% CI 23-31): 92% (95% CI 88-95%) vs. 65% (60-69%):
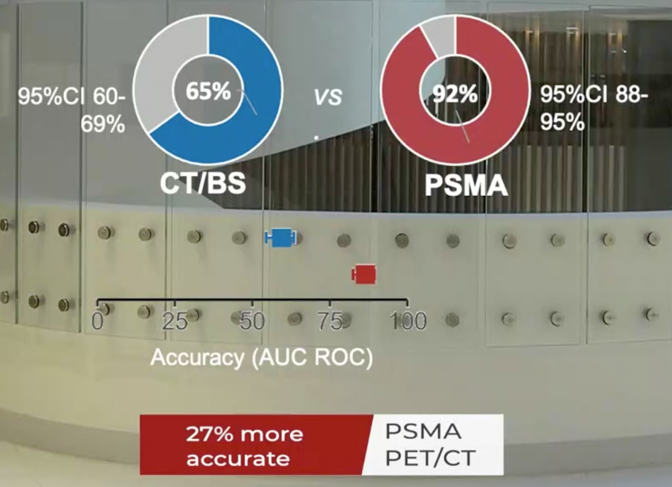
Conventional imaging had both a lower sensitivity (38% vs. 85%) and also a lower specificity (91% vs. 98%). Prior to treatment, the results of conventional imaging studies resulted in treatment change for 23 men (15%, 95% CI 10-22) while the results of PSMA PET-CT resulted in treatment change for 41 (28%, 95% confidence interval 21-36). These changes included both a transition from curative intent to palliative intent treatment in 20 patients (14%) and also a change in treatment approach in 22 (14%). Additionally, conventional imaging was associated with a higher radiation dose (19.2 mSv compared to 8.4 mSv; absolute difference 10.9 mSv, 95% CI 9.8-12.0 mSv0. PSMA PET-CT was not associated with any adverse events and reporter agreement was high for both nodal (kappa 0.87, 95% CI 0.81-0.94) and distant metastatic disease (kappa 0.88, 95% CI 0.94-0.92).
Part of the trial design was a built-in health economics perspective, and Dr. Murphy and colleagues recently published this analysis in European Urology [2]. They found that the estimated cost per scan for PSMA PET/CT was AUD$1203, which was less than the conventional imaging cost at AUD$1412.

PSMA PET/CT was thus dominant, having both better accuracy and a lower cost, resulting in a cost of AUD$959 saved per additional accurate detection of nodal disease, and AUD$1412 saved for additional accurate detection of distant metastases. Additionally, these results were most sensitive to variations in the number of men scanned for each 68Ga-PSMA-11 production run. Dr. Murphy suggests that based on the proPSMA study, PSMA PET/CT has established itself as having superior accuracy for staging compared to conventional imaging, as well as less equivocal findings, less radiation dose, greater management impact, and more cost-effective. As such, Dr. Murphy and his colleagues recommend that PSMA PET/CT should replace conventional imaging for high-risk disease staging.
With regards to PSMA PET/CT for recurrent disease, Dr. Murphy points to his group’s systematic review and meta-analysis that showed the accuracy of PSMA PET/CT in the biochemical recurrent setting stratified by PSA level:
- 0-0.19 ng/mL: 33%
- 2-0.49 ng/mL: 42%
- 5-0.99 ng/mL: 59%
- 1-1.99 ng/mL: 75%
- >2 ng/mL: 95%
The most recent EAU guidelines suggest that PSMA PET/CT should be performed if the PSA level is >0.2 ng/mL and if the results will influence subsequent treatment decisions. Dr. Murphy concluded his presentation by emphasizing that novel imaging can cause disruption and uncertainty, as new research questions will emerge. At the crux of these questions is whether earlier detection of recurrent disease and changes in management will ultimately alter downstream prostate cancer specific outcomes.
Presented by: Declan Murphy, MB, BCH, BaO, FRACS, FRCS, Urol, Professor, Consultant urological surgeon at Peter MacCallum Cancer Centre and the Royal Melbourne Hospital, Melbourne, Australia and Director of Outcomes Research at the Australian Prostate Cancer Research Centre
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.References:
1. Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020 Apr 11;395(10231):1208-1216.
2. De Feria Cardet RE, Hofman MS, Segard T, et al. Is Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Imaging Cost-effective in Prostate Cancer: An Analysis Informed by the proPSMA Trial. Eur Urol. 2020 Dec 16;S0302-2838(20)30946-5.
3. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68GA-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur Urol 2016;70(6):926-937.


