Dr. Blanchard started his presentation by emphasizing that stereotactic body radiotherapy works. A recent systematic review and meta-analysis by Lehrer et al. published in JAMA Oncol1 assessed the safety and clinical benefit of stereotactic body radiotherapy in oligometastatic cancer. Among 21 studies comprising 943 patients and 1290 oligometastases, the most common primary sites were prostate (22.9%), colorectal (16.6%), breast (13.1%), and lung (12.8%). The random-effects estimate for 1-year local control was 94.7% (95% CI 88.6%-98.6%; I2 = 90%; 95% CI 86%-94%; and τ = 0.81%; 95% CI 0.36%-2.38%).
There is also evidence for stereotactic body radiotherapy in pelvic nodal relapse. The Oligopelvis-GETUG P07 trial was first presented at the 2020 GU ASCO meeting and just published this weekend in European Urology.2 This was a multicenter phase II trial of combined salvage radiotherapy and hormone therapy (6 months of ADT) in oligorecurrent pelvic node relapsed prostate cancer. The main objective of this trial was to assess biochemical-clinical failure defined by a cluster of events including PSA progression (≥25 % and ≥ 2 ng/ml above the nadir) or clinical evidence of local or metastatic progression or post-treatment initiation of hormonal therapy or prostate cancer-related death. Among 67 patients, at 2 and 3 years, 73.1 and 45.9% of patients achieved a persisting complete response, respectively. After a median follow-up of 34 months, the 2-year progression-free survival rate was 77.6%, and the median progression-free survival was 40.1 months. With regards to adverse effects of treatment, grade 2+ two-year urinary and intestinal toxicity were only 10% and 2%, respectively. Additionally, there are trials ongoing in this disease space that are of interest:
- OLIGOPELVIS 2 (NCT03630666): Randomized phase III versus interim ADT with a primary endpoint of biochemical progression-free survival
- PEACE 5 – STORM trial: Stereotactic body radiotherapy versus elective nodal radiotherapy
Based on the APCCC 2019 meeting, the experts suggest that:
- For the majority of patients with de novo (synchronous) oligometastatic prostate cancer (based on conventional imaging or next generation imaging) who have an untreated primary tumor, half of the panelists voted for systemic therapy plus treatment of the primary tumor and focal treatment of all lesions
- For the majority of patients with oligorecurrent prostate cancer, 75% of panelists voted for systemic therapy and local treatment of all lesions
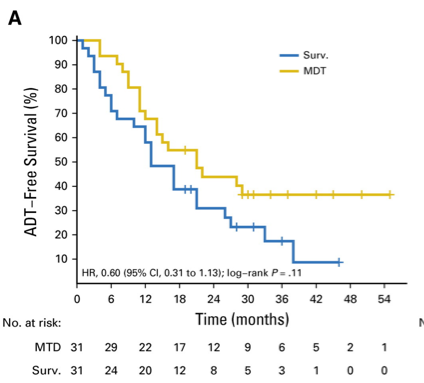
To date, the data for metastasis directed therapy for oligometastases involves small phase II trials. In the STOMP trial,3 Ost and colleagues randomly assigned 62 patients to either surveillance or metastasis-directed therapy of all detected lesions (surgery or stereotactic body radiotherapy), with a primary end point of ADT-free survival. At a median follow-up time of 3 years (IQR 2.3-3.75 years), the median ADT-free survival was 13 months (80% CI 12 to 17 months) for the surveillance group and 21 months (80% CI 14 to 29 months) for the metastasis-directed therapy group (HR 0.60, 80% CI 0.40 to 0.90; log-rank p = 0.11):
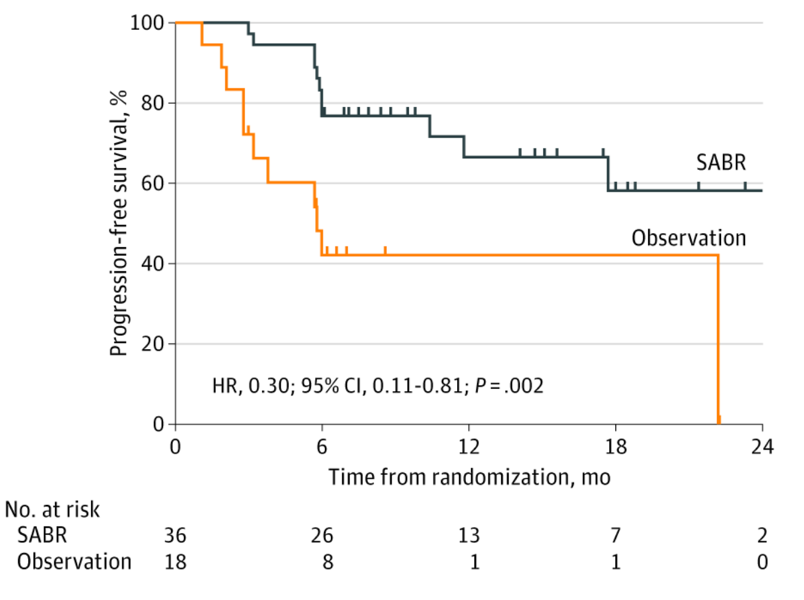
The second phase II trial published recently was the ORIOLE trial,4 randomizing 54 men in a 2:1 ratio to receive stereotactic body radiotherapy or observation. The primary endpoint for this trial was progression at 6 months, defined as a PSA increase, radiographic or symptomatic progression, ADT initiation, or death. Progression at 6 months occurred in 7 of 36 patients (19%) receiving stereotactic body radiotherapy and 11 of 18 patients (61%) undergoing observation (p = 0.005). Furthermore, treatment with stereotactic body radiotherapy improved median progression-free survival (not reached vs 5.8 months; HR 0.30, 95% CI 0.11-0.81; p = 0.002):
For those patients in the stereotactic body radiotherapy arm that had a PSMA PET-CT scan, the proportion of men with disease progression at 6 months was 5% in those who did not have any untreated lesions, compared to 38% in those who did have some untreated PSMA avid lesions (p=0.03).
As follows is a list of ongoing metastasis directed therapy trials in hormone sensitive oligometastatic prostate cancer:
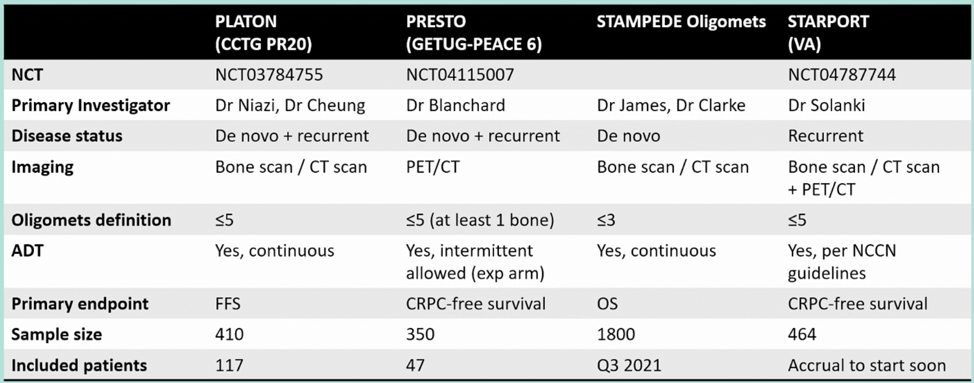
Furthermore, Dr. Blanchard emphasizes that with these ongoing trials we need (i) larger trials than STOMP and ORIOLE, (ii) more robust clinical endpoints (ie. CRPC free survival, overall survival), (iii) potential for prospective meta-analyses, and (iv) a different aim based on the imaging technique used for staging.
Dr. Blanchard concluded his presentation with the following take home messages:
- There is currently low level of evidence for metastasis-directed therapy in oligo metastatic recurrent prostate cancer
- Despite the level of evidence, metastasis directed therapy is commonly used in clinical practice
- It is important to recruit to randomized trials in order to demonstrate a magnitude of benefit, which also provides an avenue for large scale, prospective meta-analyses
Presented by: Pierre Blanchard, MD, PhD, Institut Gustave Roussy, Universite Paris-Saclay, Villejuif, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
- Lehrer EJ, Singh R, Wang M, et al. Safety and Survival Rates Associated with Ablative Stereotactic Radiotherapy for Patients with Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2021 Jan 1;7(1):92-106.
- Supiot S, Vaugier L, Pasquier D, et al. OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur Urol 2021 July 8 [Epub ahead of print].
- Ost P, Reynders D, Decaestecker K, et al. Surveillance of Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018 Feb 10;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020 Mar 26;6(5):650-659.


