(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Professor Valeria Panebianco discussed the Vesical Imaging-Reporting and Data System (VI-RADS) and its potential to replace/complement transurethral resection of bladder tumor (TURBT) for diagnosing bladder cancer.
The currently established paradigm for diagnosing bladder cancer can be summarized below. Patients with specific signs/symptoms (e.g., hematuria) undergo cystoscopic evaluation (plus cytology and cross-sectional imaging) followed by a TURBT to diagnose and stage bladder cancer.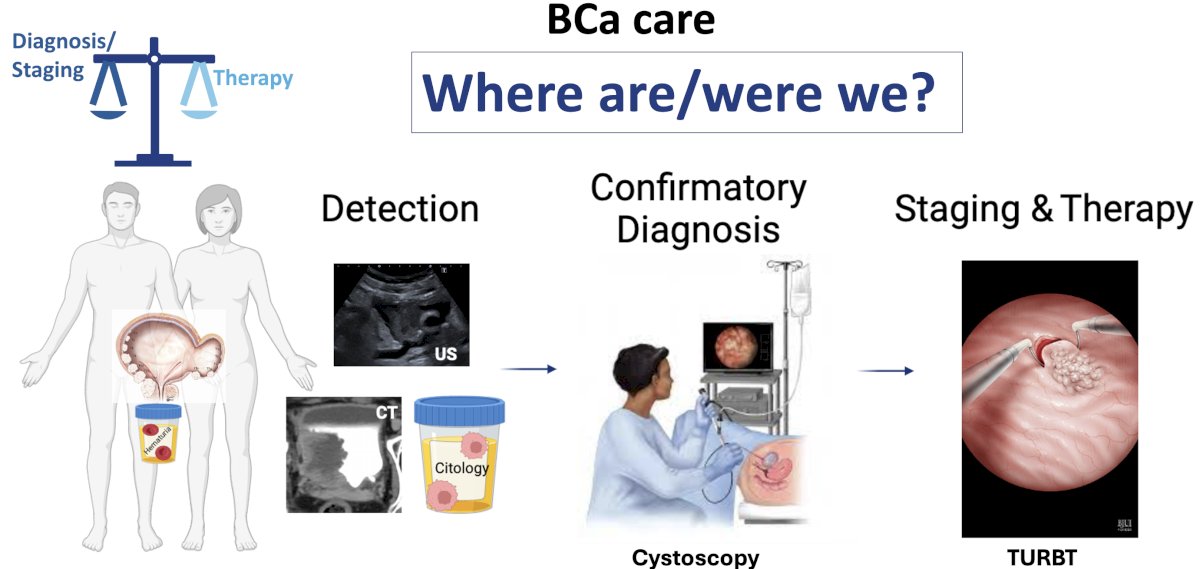
The goals of TURBT are to accurately stage bladder cancer with appropriate sampling of the detrusor muscle and to fully eradicate tumors without any residual disease, where feasible. However, there are numerous limitations to TURBT:
- Disease understaging and residual tumor
- Operator-dependent (experience, judgment, skill)
- Low sensitivity for sampling
- Residual disease (present in up to 58%)
- ‘Lack of sense’ of the tumor depth (e.g., stage T3)
- Lack of accurate complete mapping
- Bladder cancer-related costs
There is clear, strong evidence that TURBT under-stages bladder tumors. A retrospective evaluation of 279 patients who were staged as cT1 and subsequently underwent a radical cystectomy demonstrated that 48% of patients were understaged. Notably, understaged patients had significantly worse survival outcomes, highlighting the importance of accurate staging at TURBT.1
Additionally, TURBT quality varies across the world and within the UK and Europe, likely leading to inconsistencies in tumor staging and eradication/resection.
This is reflected in differences in recurrence patterns following TURBT, likely due to differences in operator performance, as opposed to underlying tumor differences.
Given these limitations, the VI-RADS scoring system was developed to define a standardized approach to the imaging and reporting of bladder cancer, evaluating the likelihood of muscle-invasive disease. This scoring system leverages the high contrast resolution of MRI allowing for an excellent differentiation of bladder wall layers. A 5-point scale is utilized as follows:
- Muscle invasion is highly likely
- Muscle invasion is unlikely to be present
- Muscle invasion is equivocal
- Muscle invasion is likely to be present
- Invasion of muscle and beyond the bladder is highly likely

This scoring system has become widely adopted, as evidenced by the 99 articles published investigating the diagnostic performance of VI-RADS, with 9 ensuing meta-analyses, and one ongoing trial in this space (Bladder Path). A review of 22 prospective or well-designed, large retrospective studies has demonstrated the following performance characteristics:
- Retrospective studies
- Sensitivity: 82–97%
- Specificity: 61.4–97%
- Area under the curve (AUC): 0.91–0.96
- Prospective studies
- Sensitivity: 78–100%
- Specificity: 78.8–99.1%
- Area under the curve (AUC): 0.83–0.96
- In the prospective study with the highest number of readers (n=5), the inter-reader agreement ranged from k = 0.70 –0.92.

How can MRI be incorporated into bladder cancer pathways? In addition to evaluating the likelihood of muscle invasion/extravesical disease, this imaging tool can be used to map the bladder, evaluating for multi-focal disease.
For patients with non-muscle invasive bladder cancer (NMIBC), the MRI and VI-RADS scoring system could be clinically implemented as follows:
- Patients with VI-RADS 1 &2 could be considered for immediate TURBT with the diagnostic cystoscopy forgone in lieu of the MRI
- Patients with high-risk T1 disease and VI-RADS 1 &2 on imaging Forgo re-staging TURBT

Conversely, for patients with likely MIBC, the MRI and VI-RADS scoring system could be clinically implemented as follows:
- VI-RADS 3-4: Sampling TURBT should be performed for confirmatory staging
- VI-RADS 5: Confirmatory biopsy is performed, with no need for a deep transurethral resection

In 2023, a Delphi consensus on the clinical application of bladder MRI and VI-RADS system was published with the following highlights:2
- MRI should be acquired and interpreted according to the VI-RADS recommendations
- MRI should always be performed before TURBT
- MRI should be used to assess response to systemic therapy to select patients for treatment
What are the guidelines recommendations for the use of MRI in the bladder cancer diagnosis/management pathway? The 2024 EAU guidelines section for “diagnostic workup in patients with confirmed MIBC” currently state:
- Always perform MRI before TURBT, if available (strength rating: weak recommendation)
- Offer MRI to assess the response to systemic therapy, which aids in the selection of patients for radical treatment, surveillance, and bladder-sparing surgery (strength rating: weak recommendation)
Current limitations to the widespread adoption of MRI include reader expertise, scanner availability, and the detection of carcinoma in situ.
Professor Panebianco concluded her presentation by noting that the appropriate use of MRIs allows for minimizing unnecessary cystoscopies and TURBTs using a precision diagnostic approach that uses the correct tools for correct and personalized therapeutic planning.

Presented by: Professor Valeria Panebianco, MD, Chairman of the ACR VI-RADS Committee, Department of Radiology, Sapienza University of Rome, Rome, Italy
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:- Ark JT, Keegan KA, Barocas DA, et al. Incidence and predictors of understaging in patients with clinical T1 urothelial carcinoma undergoing radical cystectomy. BJU Int. 2014;113(6):894-9.
- Panebianco V, Briganti A, Boellaard TN, et al. Clinical application of bladder MRI and the Vesical Imaging-Reporting And Data System. Nat Rev Urol. 2023.


