(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Drs. Joan Palou, Eva Comperat, and Tahlita Zuiverloon participated in a rapid-fire debate that addressed whether there was a role for urinary markers, other than cytology, for the surveillance of patients in the era of modern cystoscopy.
Professor Palou began by noting that there are 151,200 new cases of bladder cancer diagnosed annually in Europe. Approximately 75% of patients with urothelial carcinoma of the bladder present with disease confined to the mucosa (Ta, CIS) or submucosal layer (T1). Disease recurrence and progression are two key concerns for the management of non-muscle invasive bladder cancer (NMIBC).
He noted that there are several important considerations in the follow-up of NMIBC patients:
- Cost
- Patient distance to the hospital
- Credibility of the available tests
- Test invasiveness
- Confidence with the managing urologist
One tool that is frequently used for the diagnosis and surveillance of NMIBC patients is urinary cytology. While this test historically has been reported to have a sensitivity of 23 – 30% for low-grade disease and 90 – 95% for high-grade disease, it appears that the actual sensitivity for low-grade disease varies between 3% and 67%, compared to 37 – 95% for high-grade disease.
What about the other ‘pillar’ of surveillance – cystoscopy? Professor Palou noted that cystoscopy remains an invasive test with ~30% of patients reporting discomfort during the procedure and painful voiding up to 7 days post-procedurally. Another issue with cystoscopies is that it remains operator dependent and tumor detection rate with cystoscopy improves with the awareness of a positive urine test, highlighting the detection bias inherent with such an approach.
Over the past decades, we have witnessed the emergence of novel urinary markers that, to date, have not proven adequate to supplant cystoscopy/cytology in the surveillance of patients with NMIBC.
At this point, Professor Palou presented a case of a 75-year-old man with a history of recurrent, low-grade NMIBC. He remains an active smoker with medical comorbidities including hypertension and dyslipidemia. On surveillance cystoscopy, he was demonstrated to have a recurrent 3 cm papillary tumor located on the right lateral wall and another 1 cm lesion near the right ureteral orifice. Pre-operative cytology was positive for high-grade urothelial carcinoma. His pathology from the TURBT demonstrated LG Ta urothelial carcinoma. Muscle was present in the specimen and multiple bladder biopsies were negative for CIS.
He received a 6-week induction course of BCG plus 3 maintenance instillations. His repeat cystoscopy revealed patchy areas of erythema on both lateral walls. His urinary cytology demonstrated atypical urothelial cells and his urinary marker test was positive. At this point, Professor Palou turned the discussion over to Professors Comperat and Zuiverloon to argue against and for the use of urinary markers, respectively, in such a clinical setting.
Professor Comperat noted that when discussing the use of urinary cytology and its interpretation, we are referring to The Paris System, which was developed as an international form to standardize the reporting of urinary cytology. This system focuses on the identification of high-grade disease. This is reflected in the EAU guidelines recommendations which state that “urinary cytology has high sensitivity in high-grade tumors including carcinoma in situ (level of evidence: 2b”.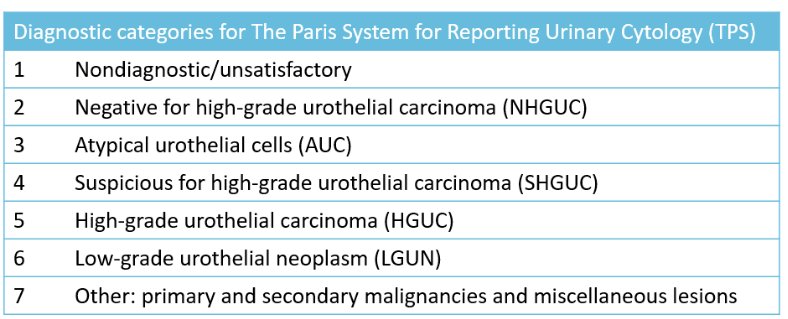
The Paris System has been shown to improve the diagnostic accuracy for detection of high-grade urothelial carcinoma in pre-operative cytology, strengthening our reliance on urinary cytology, and minimizing the role of other potential biomarkers, in this setting. Compared to prior classifications, this system reduces false-negative findings, especially in the setting of inflammation, with a negative predictive value of 80% using The Paris System.1 Based on the current evidence, Professor Comperat concluded that in instances where urinary cytology is reported as negative for high-grade urothelial carcinoma by The Paris System, there is a low probability of high-grade urothelial carcinoma, highlighting the potential diagnostic benefit of The Paris System.
What about the use of cytology in the post-BCG setting? It is clear that prior BCG has a significant impact on the interpretation of cytology findings, likely relating to underlying cellular degeneration and reactive atypia, and this is why clear documentation of prior treatment history is crucial for the reading pathologist. There is also debate whether to lower the threshold for a ‘positive’ cytology in the post-BCG setting from ‘high-grade urothelial carcinoma’ to ‘suspicious for high-grade urothelial carcinoma’, as this improves the sensitivity for detection of disease from 45% to 75%, at the cost of a specificity decrease from 91% to 79%. Interestingly, even among patients with ‘false positive’ cytologies (i.e., negative histology from enhanced photodynamic TURBTs and a positive cytology at time of negative histology), almost half of such patients were eventually diagnosed with high-grade recurrence during surveillance. As such, the investigators concluded that BCG therapy has a short-term adverse impact on the efficacy of urinary cytology. However, after BCG therapy, cases classified as suspicious for high-grade urothelial carcinoma should be considered positive. Importantly, patients with false-positive cytology findings should be closely monitored.2
While there are limitations inherent to urinary cytology for the detection of variant morphology and divergent differentiation, Professor Comperat argued that the real value of urinary cytology is for the detection of high-grade urothelial carcinoma and not for providing histologic diagnosis. The value of urinary cytology has been further reinforced by a recent study from Portugal that demonstrated that urinary cytology, when classified using The Paris System, is extremely specific (98%), with only one false positive result among 1,180 evaluable tests.3
She concluded by noting that, to date, there is no method capable of outperforming urinary cytology. Therefore, performing urinary cytology is crucial to ensure optimal oncologic outcomes for patients with NMIBC.
Next, Dr. Zuiverloon argued in favor of urinary markers in this setting. Going back to the case presentation, she noted that using the EAU risk calculator, the 1-, 5-, and 10-year risks of progression for this patient were 4, 10, and 14% respectively.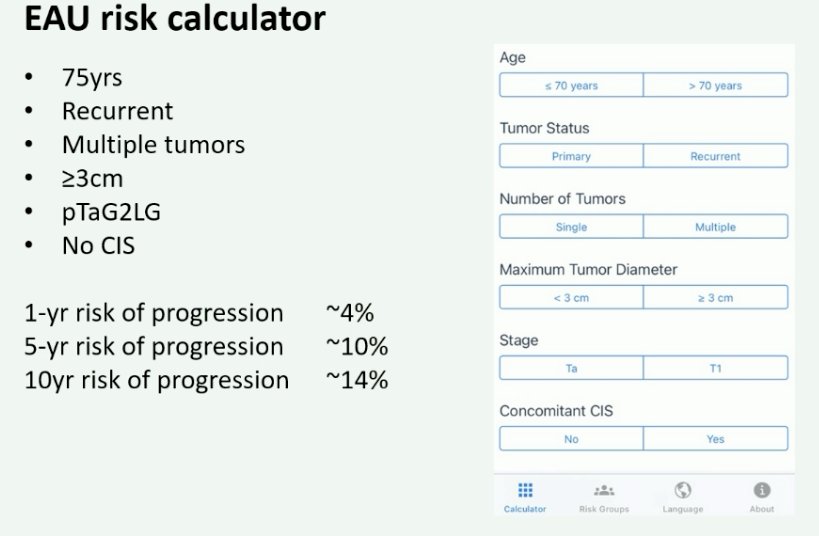
In this patient, the cytology was atypical. She noted that patients with low-grade disease, such as this patient, are likely to have low-grade recurrences in the future. Urinary cytology is limited in this setting, as its sensitivity for low-grade disease detection is 3 to 67% (~16% in prospective series). Additionally, the performance of cytology is influenced by prior intravesical therapies received such as BCG, as was noted in the case presented. There is also significant interobserver variability with regard to the interpretation of cytology results, specifically in the follow-up setting. There are frequently indeterminate findings, including atypia as in this case. Importantly, this test cannot be performed at home due to processing issues with home-based testing, thus requiring patients to present to a healthcare facility to undergo such testing.
Given these limitations, why would we use urinary markers instead? We know there are high costs associated with the follow-up of NMIBC patients, and this is strongly related to the frequency of cystoscopies and follow-up visits. As such, urinary biomarkers have the potential to reduce the frequency, and thus costs, of such tests. Cystoscopy is also frequently a painful, uncomfortable test with >30% of patients reporting pain and discomfort during the procedure. There are also important diagnostic limitations with cystoscopy. While patients expect/demand 95% sensitivity with this test, the reality is that the sensitivity of cystoscopy is only 87 to 100%. We also know that a positive urine marker test improves tumor detection by the performing urologist (i.e., we look better) due to an inherent detection bias. Dr. Zuiverloon argued that all these reasons provide support for considering urinary biomarkers in this setting, particularly in the patient presented who has low-grade disease and is likely to have low-grade recurrences, where the main issue is disease recurrence, as opposed to disease progression. She argued that any urinary marker test in this setting must have a high negative predictive value for ruling out the presence of high-grade disease, whereby test negative patients may have their cystoscopy omitted, with a corresponding high sensitivity for high-grade disease.
How do commercially available urinary marker tests perform? She noted that the negative predictive values for all these tests for the detection of any recurrences are all in excess of 80%.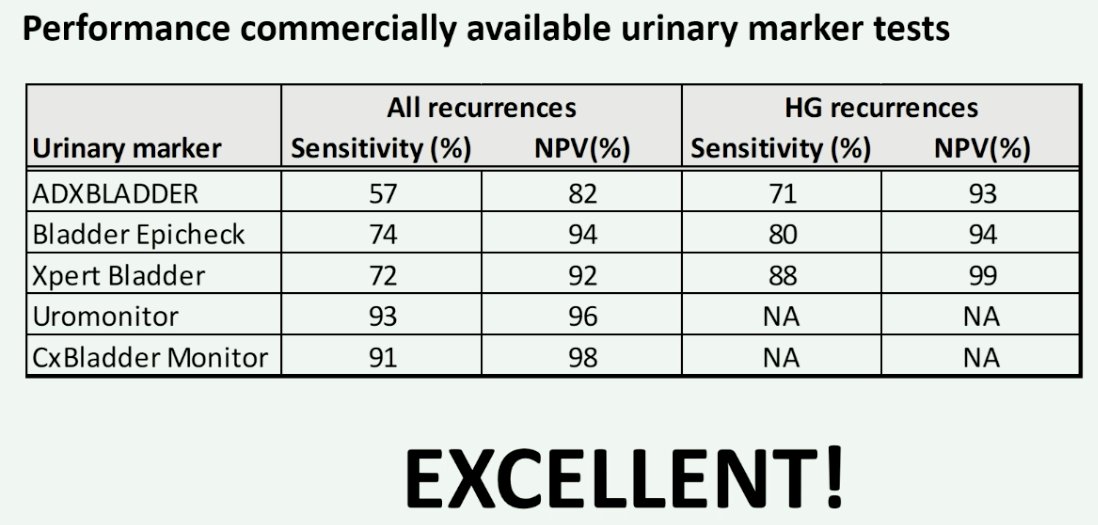
In light of this evidence, the EAU states that although novel urinary biomarkers have not been tested in randomized controlled trials, they have high sensitivities and negative predictive values in the referenced studies for high-grade disease with evidence that these biomarkers may approach the sensitivity of cystoscopy. These tests may replace and/or postpone cystoscopy as they may identify the rare high-grade recurrences occurring in low/intermediate NMIBC.
She concluded by noting that urinary markers have the ability to:
- Provide additional information, compared to cystoscopy/cytology, by detecting personalized mutations that may signal an anticipatory effect, particularly among patients with negative cystoscopy/cytology and positive urinary marker tests
- Reduce costs by replacing cystoscopy and cytology in select circumstances
- Improve the sensitivity of cystoscopy by ‘making us look harder’
- Allow for home-based testing
Presented by:
- Professor Joan Palou MD, PhD, Chairman, Department of Urology, Fundació Puigvert, Universitat Autònoma de Barcelona, Barcelona, Spain
- Professor Eva Comperat, MD, PhD, Chair of Uropathology at the Medical University of Vienna and Head of the Department of Pathology at the L'Assistance Publique-Hôpitaux de Paris, Hôpital Tenon, Sorbonne University, Paris, France
- Dr. Tahlita Zuiverloon, MD, PhD, Assistant Professor, Department of Urology, Erasmus University, Rotterdam, The Netherlands
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- Yamasaki M, Taoka R, Katakura K, et al. The Paris System for reporting urinary cytology improves the negative predictive value of high-grade urothelial carcinoma. BMC Urol. 2022;22(1):51.
- Hermans J, Jokisch F, Volz Y, et al. Impact of bacillus Calmette-Guerin intravesical therapy on the diagnostic efficacy of The Paris System for Reporting Urinary Cytology in patients with high-grade bladder cancer. Cancer Cytopathol. 2022;130(4): 294-302.
- Lobo J, Lobo C, Leca L, et al. Evaluation of the Implementation and Diagnostic Accuracy of the Paris Classification for Reporting Urinary Cytology in Voided Urine Specimens: A Cyto-Histological Correlation Study in a Cancer Center. Pathobiology. 2023;90(4): 233-240.


