Illustrated below is a summary of the use of MRI for patients with underlying MIBC. While MRI was initially limited by significant over-staging of bladder cancer, the introduction of the Vesical Imaging-Reporting and Data System (VI-RADS) scoring system altered the imaging paradigm for this disease. Several validation studies have since demonstrated improved performance characteristics for MRI. This imaging tool and scoring system have now been adopted in MRI-reliant pathways (BladderPath) that aim to expedite the time from diagnosis to intervention in patients with possible muscle invasive disease. Additionally, we are now seeing the emergence of modifications to the VI-RADS system, such as the nacVI-RADS (assess tumor response to neoadjuvant chemotherapy) and another to assess treatment response to immune checkpoint inhibitors.

Results from the PURE-01 trial of neoadjuvant pembrolizumab in MIBC were used to evaluate whether MRI can be used as a non-invasive response assessment tool. Using a three-stage approach (T1- and T2-weighted images, diffusion weighted imaging [DWI], and then dynamic contrast enhancement [DCE] to detect residual disease), Dr. Necchi and colleagues were able to demonstrate that for 37 patients with evidence of radiologic complete response, 62 – 73% of patients had no evidence of pathologic residual disease (area under the curve [AUC] of 0.74 – 0.76).1
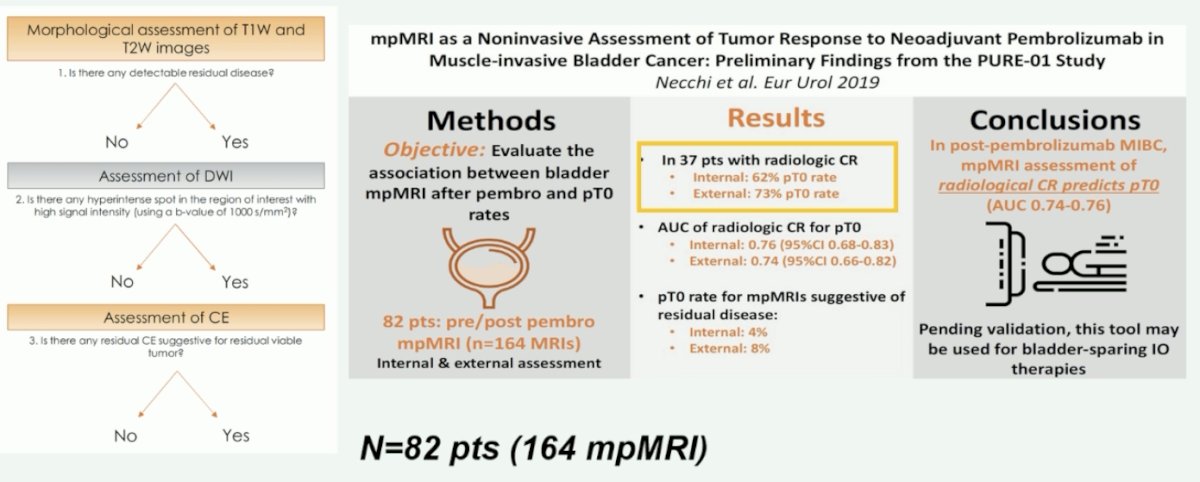
Is there a way to decrease the resources required and costs associated with the use of MRI in this setting to make it more cost-effective and widely available? One such option may be biparametric MRI. An ad hoc analysis of the PURE-01 study published in 2021 demonstrated that biparametric imaging (i.e., without the DCE component) has an equivalent AUC to multiparametric imaging (using the three components) for the prediction of ypT0N0 (AUC of 0.74 for both) and ypT≤1N0 disease (AUC of 0.87 for both).2
Is there a role for combining imaging modalities to improve upon their individual performance characteristics? The performance of bladder PET/MRI is being prospectively evaluated in the European SOGUG-NEOWIN trial of neoadjuvant erdafitinib + cetrelimab for patients with cisplatin-ineligible MIBC.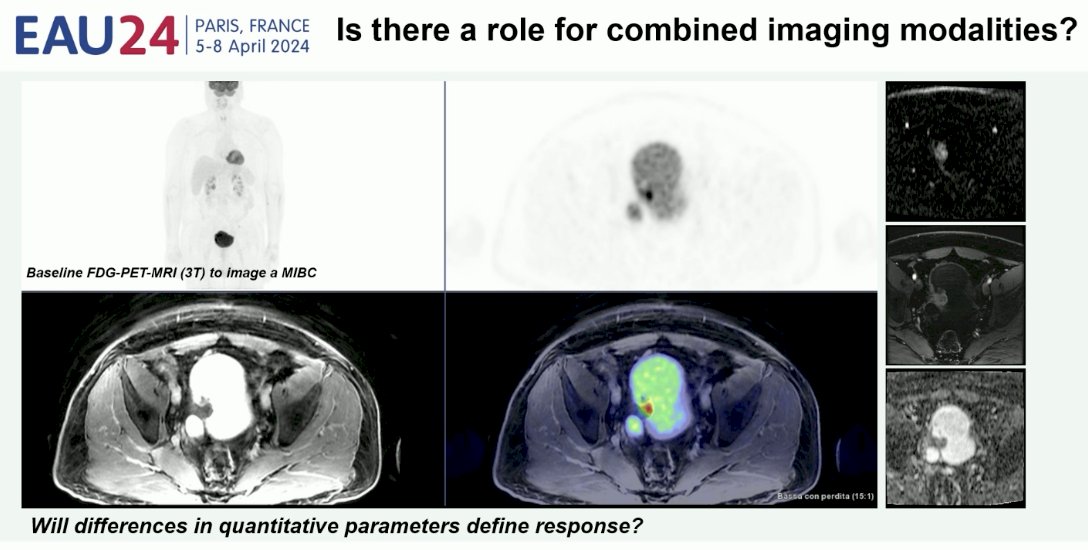
The nacVI-RADS criteria were developed in 2022 using data from 10 patients with non-metastatic MIBC who received neoadjuvant chemotherapy and underwent mpMRI before staging resection and after the chemotherapy cycles. NacVI-RADS categorically defines complete radiologic response, based on prior VI-RADS score, presence of residual disease, tumor size, and infiltration of the muscularis propria.3 This scoring system is currently being validated in a cohort of 220 patients in the post-pembrolizumab setting.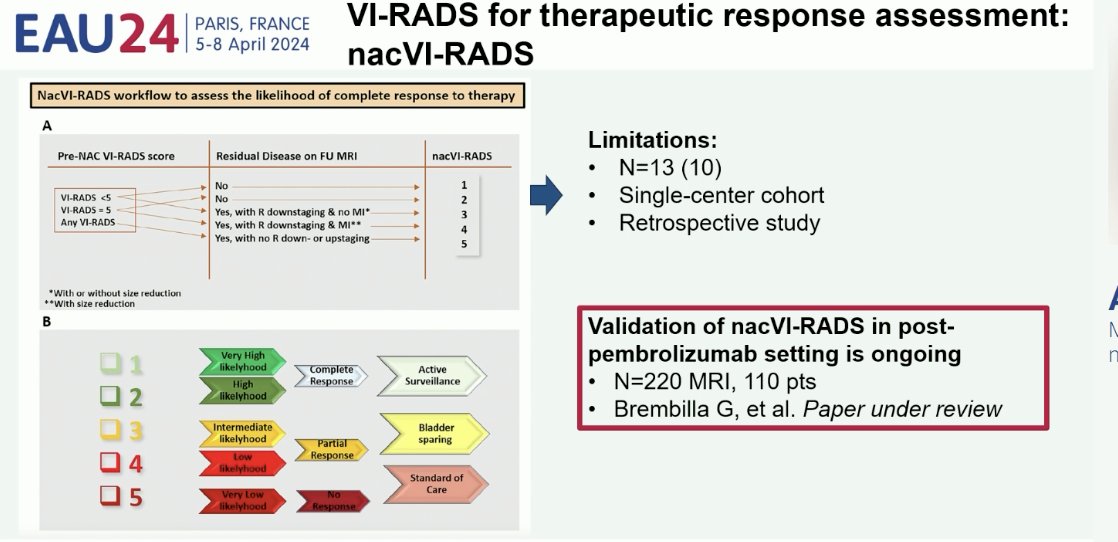
In 2024, Dr. Necchi’s group introduced an additional modification of the VI-RADS scoring system, whereby a VI-RADS 0 category was introduced to indicate no evidence of disease on MRI. This is of particular importance given the increased interest in adopting bladder-sparing therapies in patients with evidence of a complete clinical response following radical TURBT and systemic therapy. Notably, 33% of patients with VI-RADS 4-5 disease pre-pembrolizumab were downgraded to VI-RADS 0-3 disease following pembrolizumab.4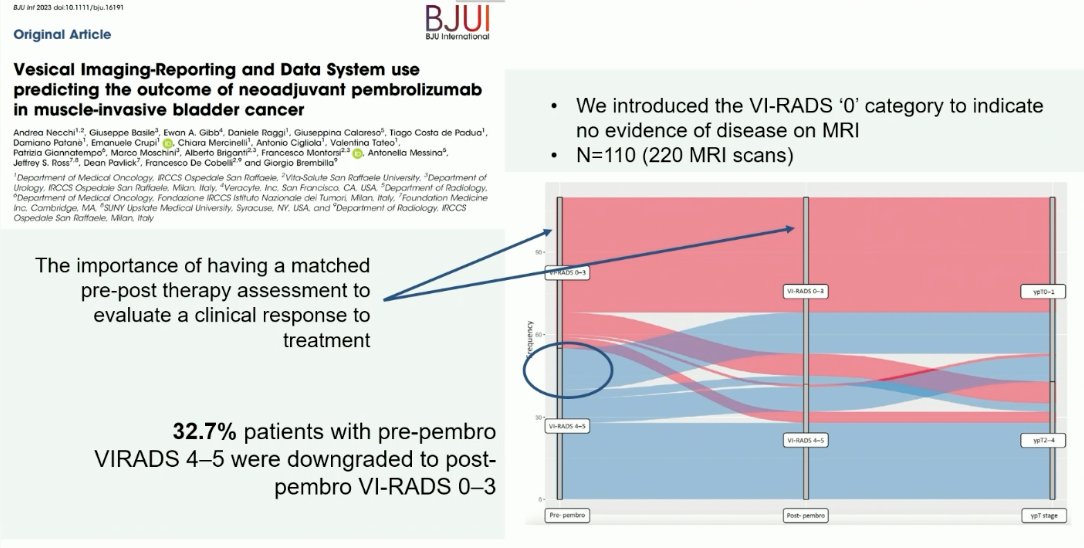
There also appear to be differences in gene signatures between patients with pre-therapy VI-RADS scores of 0–3 versus 4–5 from the PURE-01 trial. As demonstrated below, patients with lower VI-RADS scores (0–3) have higher Interferon Alpha and Gamma expression and decreased angiogenesis.4
Ultimately, one of the goals of incorporating such imaging in the MIBC management paradigm is to better detect the complete responders to systemic therapy who could be spared radical cystectomy for their muscle-invasive disease. What is clear is that the VI-RADS score is prognostic of clinical outcomes in patients with muscle-invasive disease. This has been demonstrated in both radical cystectomy cohorts from the PURE-01 trial and among patients from the bladder-sparing HCRN GU-16-257 trial.5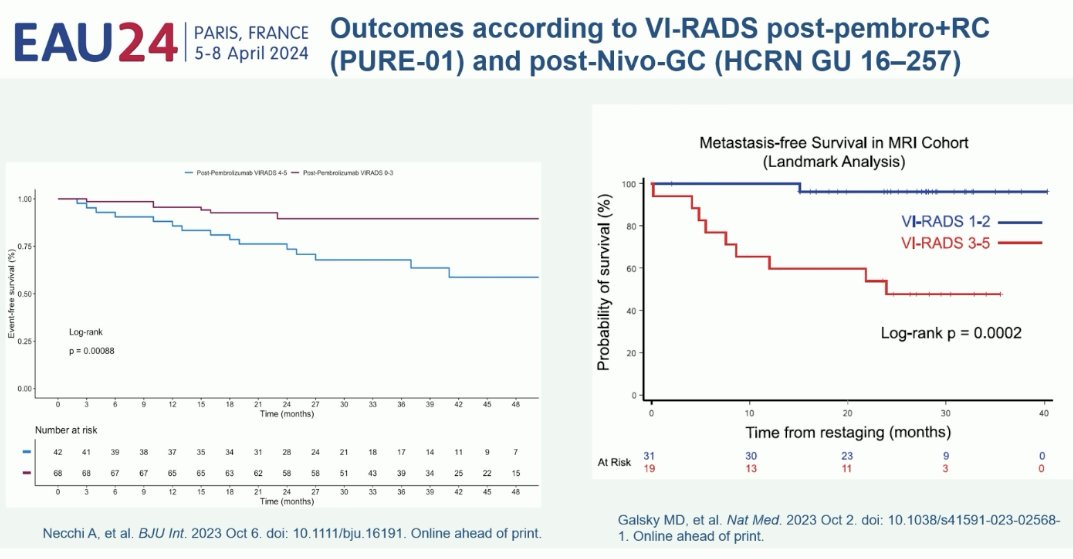
Dr. Necchi concluded his presentation as follows:
- Bladder MRI and VI-RADS use have shown incremental possibilities to predict a complete response to neoadjuvant therapy, but we need to improve the accuracy to identify the outlier responders.
- Composite models that incorporate local (MRI/biopsy/urine cytology/utDNA) and systemic therapy responses (ctDNA) may be a solution.
- Yet uncertainties exist regarding the management of residual disease (radically-resected T2 residual disease after reTURBT, residual pTa/T1 HG tumors, etc.).
- These considerations may change according to the type of neoadjuvant therapies and possible maintenance systemic therapy that are offered to the patients within trials.
- Trials of patient allocation for bladder preservation based on complete response are an intelligent way of addressing patients' concerns regarding radical cystectomy, but we need harmonization of design and outcomes.
- There is a need for a consensus on what constitutes cure and curative intent in MIBC given the emergence of novel, non-surgical therapy options to support adequate clinical trial designs and healthcare decision-making.
Presented by: Professor Andrea Necchi, MD, Director of GU Medical Oncology, San Raffaele Hospital and Scientific Institute, Milan, Italy
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:- Necchi A, Bandini M, Calareso G, et al. Multiparametric Magnetic Resonance Imaging as a Noninvasive Assessment of Tumor Response to Neoadjuvant Pembrolizumab in Muscle-invasive Bladder Cancer: Preliminary Findings from the PURE-01 Study. Eur Urol. 2020;77(5): 636-643.
- Bandini M, Calareso G, Raggi D, et al. The Value of Multiparametric Magnetic Resonance Imaging Sequences to Assist in the Decision Making of Muscle-invasive Bladder Cancer. Eur Urol Oncol. 2021;4(5): 829-833.
- Pecoraro M, Del Giudice F, Magliocca F, et al. Vesical Imaging-Reporting and Data System (VI-RADS) for assessment of response to systemic therapy for bladder cancer: preliminary report. Abdom Radiol (NY). 2022;47(2): 763-770.
- Necchi A, Basile G, Gibb EA, et al. Vesical Imaging-Reporting and Data System use predicting the outcome of neoadjuvant pembrolizumab in muscle-invasive bladder cancer. BJU Int. 2024;133(2): 214-222.
- Galsky MD, Daneshmand S, Izadmehr S, et al. Gemcitabine and cisplatin plus nivolumab as oan rgan-sparing treatment for muscle-invasive bladder cancer: a phase 2 trial. Nat Med. 2023;29: 2825-2834.


