(UroToday.com The 2024 European Association of Urology (EAU) annual meeting featured a game changing session on prime time for adjuvant treatment in locally advanced bladder cancer, and a presentation by Dr. Thomas Powles discussing clinical outcomes in patients with high-risk, post-cystectomy muscle-invasive bladder cancer with persistent ctDNA- status on serial testing in the IMvigor011 trial.
Previous data suggest that ctDNA may be able to identify patients with muscle invasive bladder cancer who are at risk of relapse after surgery. In the phase III IMvigor010 study, landmark ctDNA testing in a high-risk muscle invasive bladder cancer population discriminated ctDNA+ from ctDNA- patients, but that ~30% of ctDNA- patients experienced a DFS event. IMvigor011 is a global, double-blind, randomized phase III study evaluating the efficacy of atezolizumab (anti-PD-L1) versus placebo in patients with high-risk muscle invasive bladder cancer who are ctDNA+ post-cystectomy. At the 2024 EAU annual meeting, Dr. Powles presented the analysis examining clinical outcomes in patients with persistent ctDNA- status on serial ctDNA monitoring from the IMVigor011 surveillance cohort. The IMvigor011 trial design is as follows: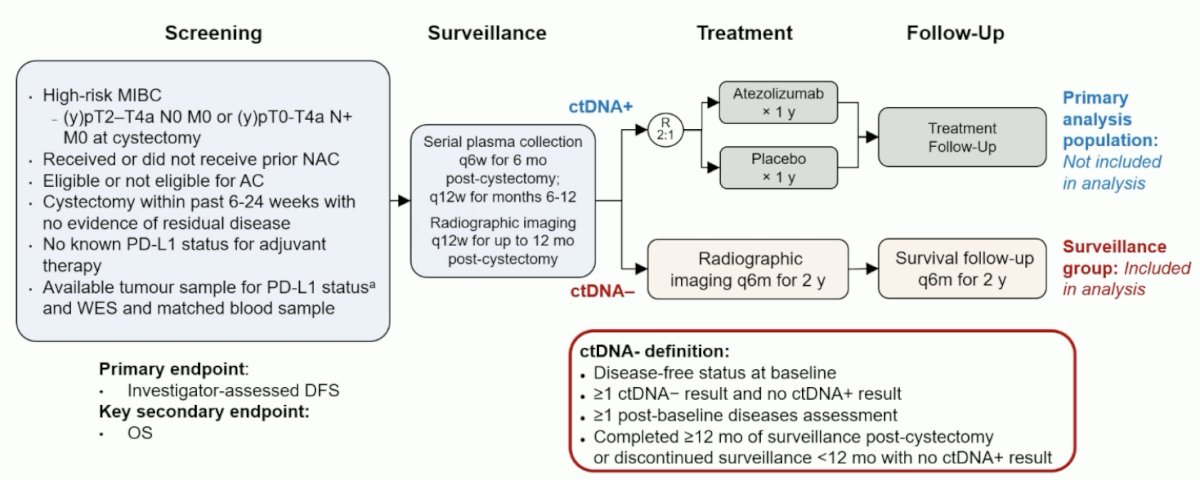
Overall, there were 286 patients that were ctDNA-, with 115 that have follow-up ongoing, thus 171 meeting criteria and included in the analysis: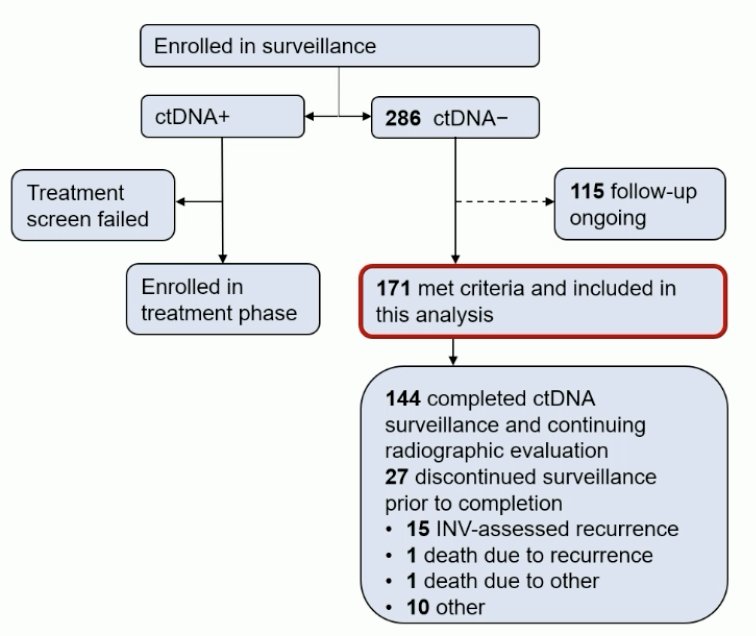
The baseline characteristics among the ctDNA- population is highlighted as follows: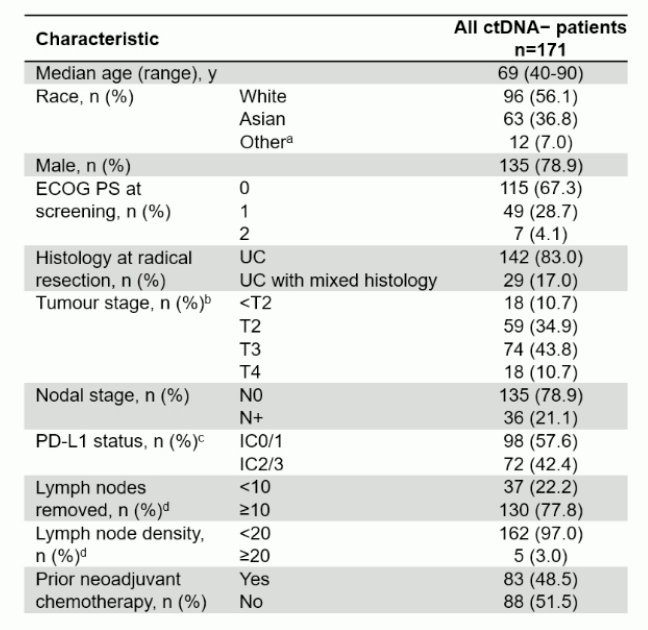
Over a median follow-up of 16.3 months (IQR 11.6-19.3), there were 17 DFS events in the ctDNA- population. The 12 month DFS rate was 92% and was 88% at 18 months: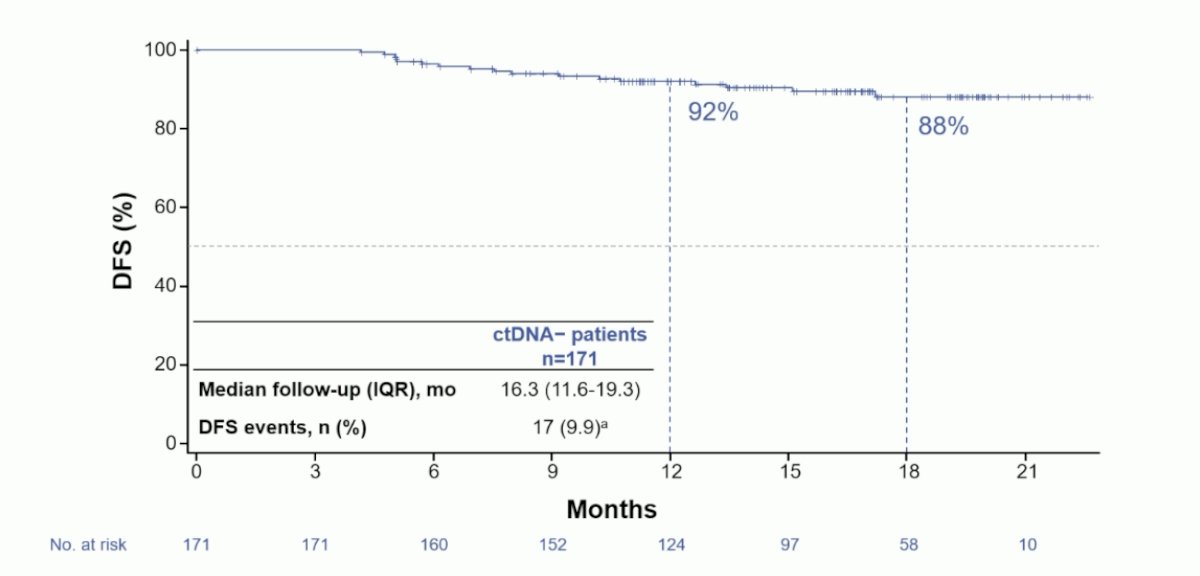
In the PD-L1 subgroups among the ctDNA- negative population, median DFS was not reached in either the PD-L1 IC0/1 or PD-L1 IC2/3 cohort: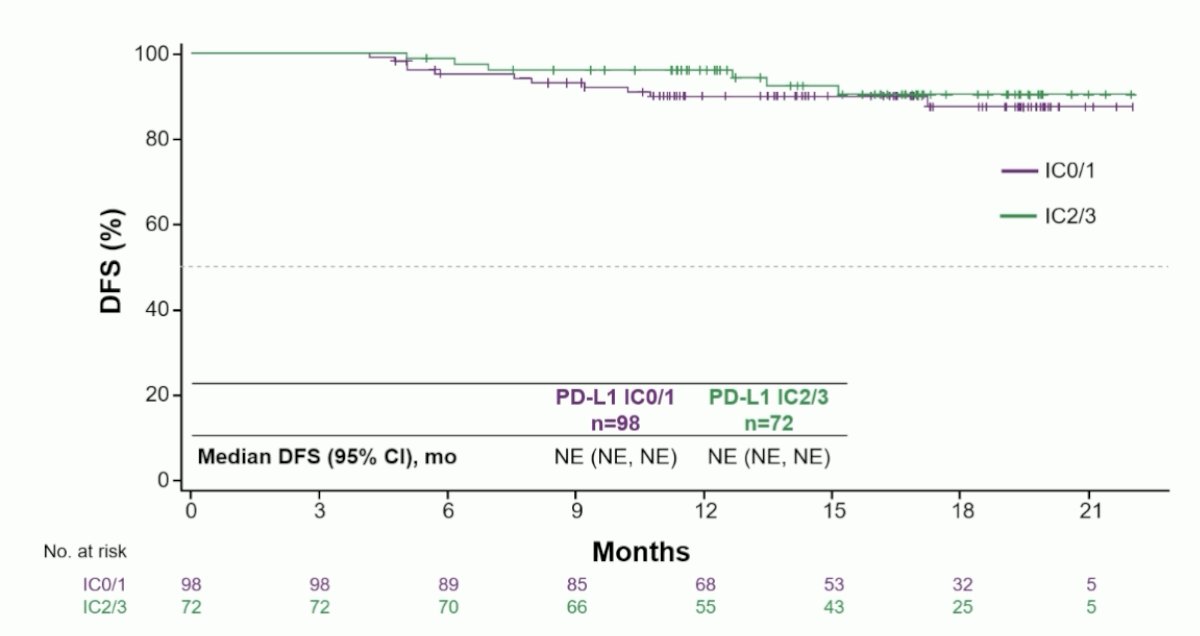
With regards to overall survival, over a median follow-up of 16.3 months (IQR 11.6-19.3), there were 2 events. The 12 month OS rate was 100% and the 18 month OS rate was 98%. A comparison of the characteristics of ctDNA- patients with recurrence versus those without disease recurrence is as follows: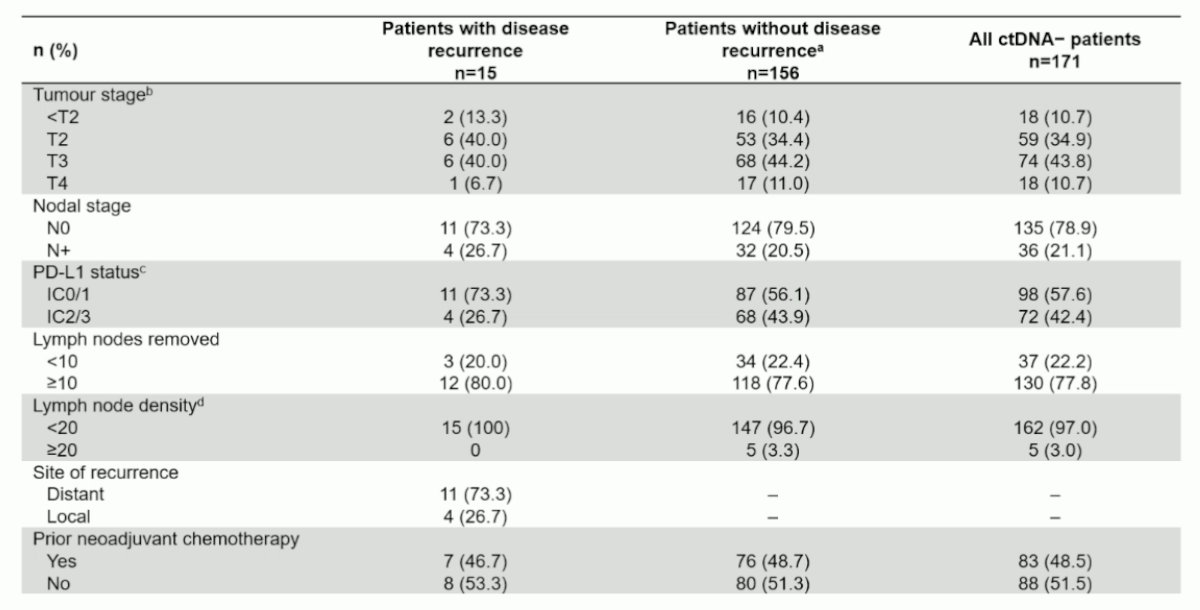
Dr. Powles concluded his presentation discussing clinical outcomes in patients with high-risk, post-cystectomy muscle-invasive bladder cancer with persistent ctDNA- status on serial testing in the IMvigor011 trial with the following conclusions:
- This analysis suggests that serial ctDNA testing may have greater clinical utility than landmark ctDNA testing as a risk stratification tool
- These data lend increasing confidence that patients with high risk muscle invasive bladder cancer who have persistent ctDNA- status after cystectomy may be spared from adjuvant treatment
- This analysis was limited to ctDNA- patients and suggests that ctDNA status selects for patients with favorable clinical prognosis regardless of PD-L1 status and pathologic staging at cystectomy
Presented by: Professor Thomas Powles, MD, Director, Barts Cancer Centre, London, UK
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024


