(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a game changing session on prime time for adjuvant treatment in locally advanced bladder cancer, and a presentation by Dr. Matthew Galsky discussing extended follow-up from the CheckMate 274 trial, including the first report of overall survival outcomes.
Despite standard of care radical cystectomy with or without neoadjuvant cisplatin-based chemotherapy, a significant number of patients with muscle invasive urothelial carcinoma experience disease recurrence within 3 years of surgery. Nivolumab became the standard of care adjuvant treatment for patients with high risk muscle invasive urothelial carcinoma after radical surgery based on the initial results from the phase 3 CheckMate 274 trial.1 CheckMate 274, which assessed adjuvant nivolumab for patients with high risk muscle invasive urothelial carcinoma after radical surgery, met both of its primary endpoints: with a minimum follow-up of 5.9 months (median follow-up, 20.9 months for nivolumab and 19.5 months for placebo), adjuvant nivolumab improved disease free survival versus placebo in the intention to treat population (HR 0.70, 98.22% 0.55-0.90) and in patients with tumor PD-L1 expression >= 1% (HR 0.55, 98.72% CI 0.35-0.85). At the EAU 2024 annual meeting, Dr. Galsky presented extended follow-up results from CheckMate 274, including the first report over overall survival from this trial.
CheckMate 274 was a phase 3, randomized, double-blind, multicenter study of one year of adjuvant nivolumab versus placebo for high-risk muscle invasive urothelial carcinoma (ypT2-ypT4a or ypN+ who had neoadjuvant cisplatin chemotherapy, or pT3-pT4a or pN+ without prior neoadjuvant cisplatin chemotherapy and not eligible/refuse adjuvant cisplatin chemotherapy) with the following trail design: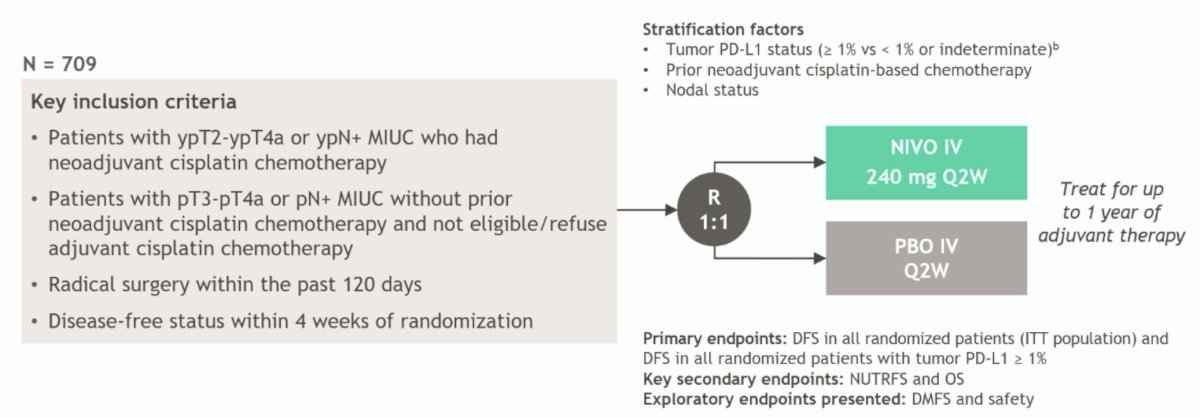
The primary endpoint was disease-free survival, which continued to show a benefit for nivolumab versus placebo in the intention to treat population (HR 0.71, 95% CI 0.58-0.86) and the PD-L1 > 1% (HR 0.52, 95% CI 0.37-0.72) populations:
Interim overall survival data favored nivolumab versus placebo in the intention to treat analysis (HR 0.76, 95% CI 0.61-0.96) and tumor PD-L1 > 1% populations (HR 0.56, 95% CI 0.36-0.86):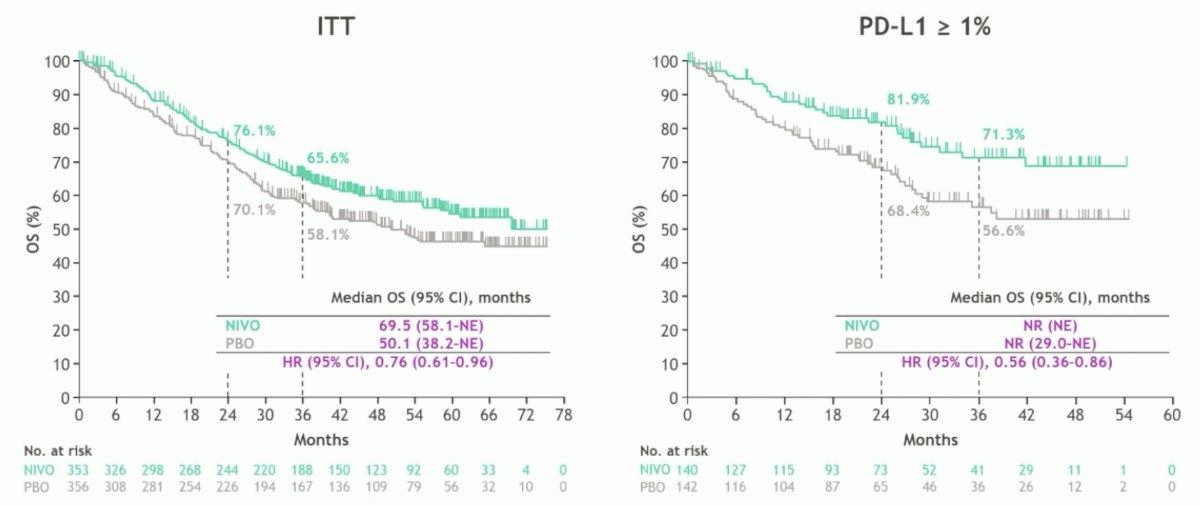
Overall survival by subgroup in the intention to treat population also generally favored the adjuvant nivolumab arm, particularly in the N+ and variant histology patients: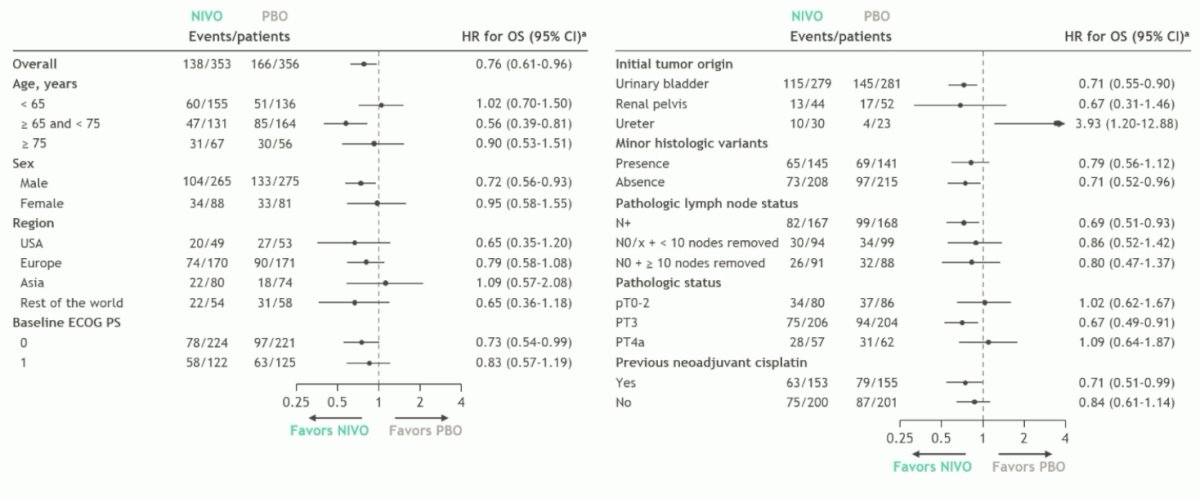
Non-urinary tract free survival (key secondary endpoint) and distant metastasis free survival (key exploratory endpoint) also favored the adjuvant nivolumab arm in the intention to treat and PD-L1 >1% population of patients: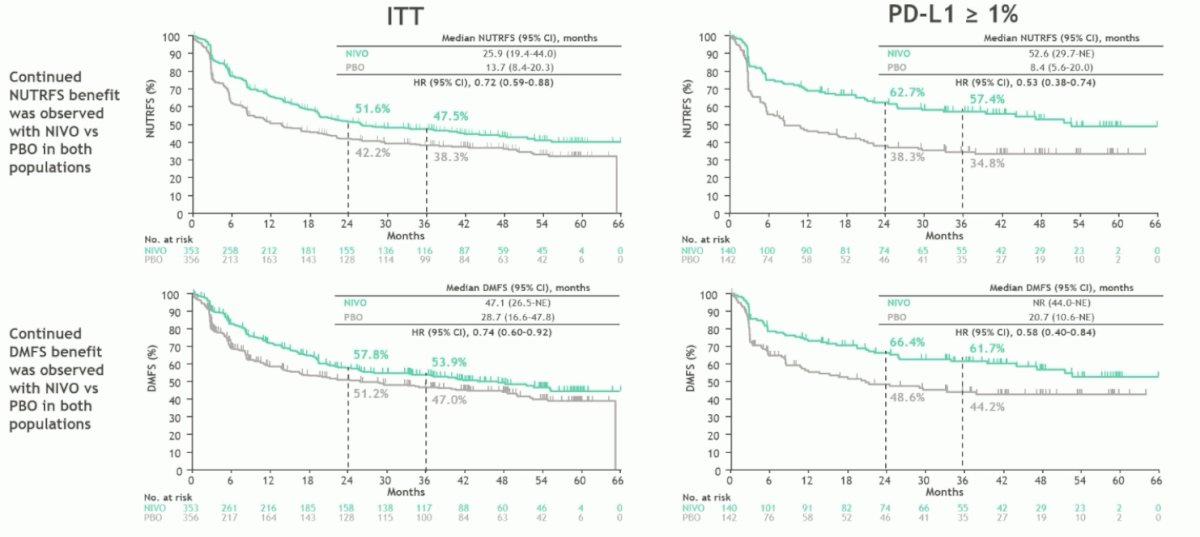
With regards to safety, any grade treatment related adverse events were identified in 79% of patients receiving nivolumab versus 56% in the placebo arm, also with higher Grade 3+ adverse events in the nivolumab arm (18% vs 7%). Common adverse events include pruritus, fatigue, diarrhea, and rash: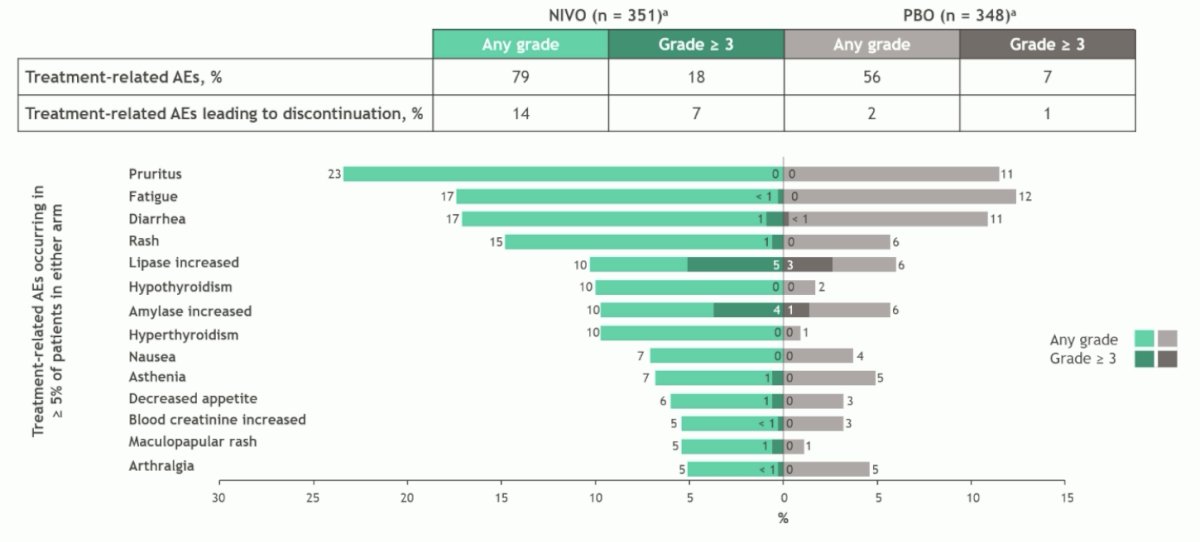
Dr. Galsky concluded his presentation discussing extended follow-up from the CheckMate 274 trial with the following conclusions:
- With extended follow-up in CheckMate 274, adjuvant nivolumab continued to show disease free survival, non-urinary tract recurrence free survival, and distant metastasis free survival benefit versus placebo in both the intention to treat and PD-L1 >=1% populations
- The overall survival data from the interim analyses favored adjuvant nivolumab versus placebo:
- In the intention to treat population: median overall survival reached 69.5 months with nivolumab versus 50.1 months with placebo (HR 0.76, 95% CI 0.61-0.96)
- In the PD-L1 >1% population: median overall survival was not reached with either treatment (HR 0.56, 95% CI 0.36-0.86); 36-month overall survival rates were 71.3% with nivolumab versus 56.6% with placebo
- There was a trend in overall survival benefit with nivolumab among prespecified subgroups of intention to treat patients
- Continued follow-up of overall survival is ongoing
- There were no new safety signals identified since the previous analysis
- These results provide additional support for adjuvant nivolumab as a standard of care for high risk muscle invasive urothelial carcinoma after radical resection, potentially providing an opportunity for a curative outcome
Presented by: Professor Matthew Galsky, MD, Icahn School of Medicine, Urology Director of Genitourinary Medical Oncology, Mount Sinai, New York, NY
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.


