(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Drs. Babjuk, Chen, and Speiss participated in a rapid-fire debate discussing how to manage a patient with frequent recurrences of low-grade papillary tumors in the bladder, despite intravesical chemotherapy and BCG therapy.
Professor Babjuk began with a case presentation of a 71-year-old female patient who was diagnosed with HG T1a disease in January 2018. Her re-staging TURBT 1 month later was negative. She proceeded to receive induction BCG x 6 with evidence of papillary recurrences in May of 2018. The TURBT revealed LG Ta disease. Professor Babjuk noted that this is not a unique situation whereby 20% of Ta recurrences 3 months following a high-grade diagnosis are found to be low grade.1
She subsequently received BCG for an additional 1 year with subsequent cystoscopies and cytologies demonstrating no evidence of recurrence. In January 2020, she was found to have a 5 mm papillary recurrence, with TURBT pathology demonstrating LG Ta disease. This recurred 3 months later with numerous lesions identified as LG Ta as well. She subsequently received 6 intravesical instillations of mitomycin and again in August 2020 was found to have multiple small papillary recurrences. At the current time, does this patient benefit more from an aggressive approach with TURBT and additional intravesical therapy, given her initial high-grade diagnosis, or should she be considered for treatment de-intensification with office fulguration and possible surveillance given her repeated low-grade recurrences?
At this point, Dr. Chen began his argument in favor of ‘staying the course’ with TURBT and additional intravesical therapy. Dr. Chen began by emphasizing that the primary index tumor was high-risk to begin with. Based on the EORTC risk calculator output, the probability of disease recurrence at 1 and 5 years is as high as 38% and 62%, with corresponding risks of progression of up to 17% and 45%, respectively. Additionally, using a risk calculator derived from a large cohort of patients (n=2,926) from two community-based, integrated health systems published in 2021, it appears that the 5-year risk of HG T1 or T2 disease recurrence or progression is 21%.2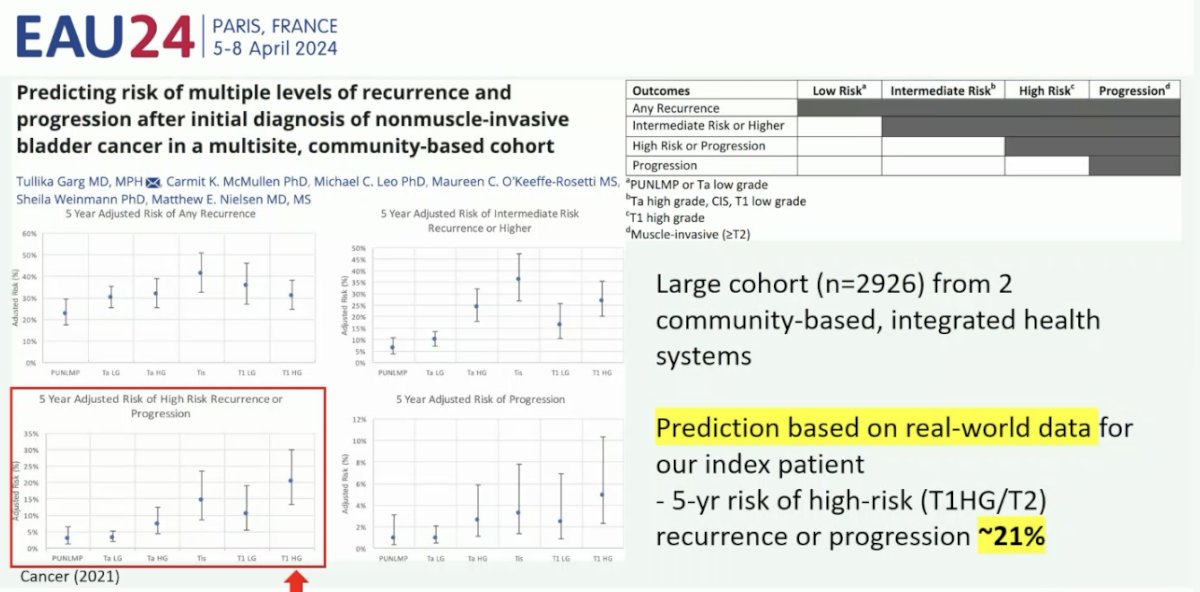
Why not consider TURBT for this patient? By all accounts, this appears to be a relatively fit 77-year-old patient with high-volume disease. TURBT is a low-morbidity procedure and is often performed as a day case. Additionally, TURBT provides the best evaluation of disease progression, and complete resections may decrease the frequency of recurrences.
And is the alternative any better? Chemoablation has not been demonstrated to be better than TURBT. The DaBlaCa-13 study was a randomized, controlled trial that was conducted in two urological departments in Denmark from January 2018 to August 2021. In total, 120 patients with a history of Ta low- or high-grade NMIBC were included upon recurrence. The intervention group received intravesical MMC (40 mg/40 mL) three times a week for 2 weeks and TURBT or office biopsy only if the response was incomplete. The control group received TURBT or office biopsy and 6 weekly adjuvant instillations. Significantly fewer patients required a procedure in the intervention group (71% versus 100%, p<0.001). However, the 12-month recurrence-free survival was 36% in the intervention group, compared to 43% in the control group (p=0.50).3
Why should we continue intravesical therapy in such a case? This patient has evidence of recurrent, multifocal LG Ta disease, which is considered International Bladder Cancer Group (IBCG) intermediate risk disease. As such, this patient has not experienced BCG failure. Given her history, she would be recommended for intravesical treatment for up to 1 year.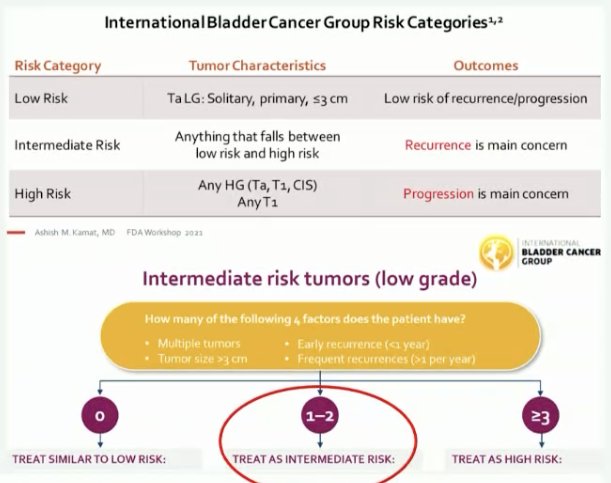
Dr. Chen emphasized that we cannot assume that these low-grade recurrences are actually benign. While low-grade recurrences experienced during BCG treatment are associated with less than half the progression events observed with high-grade recurrences, the estimated 5-year progression rates remain significant at 14.4% with such low-grade recurrences.4 Additionally, this patient has concerning LG recurrence features that warrant additional intravesical treatment, namely multifocal recurrences with increasing frequency (>1/year). Per the IBCG, this increased frequency of intervention should prompt consideration of further intravesical therapy.
There also may be a role for addition of immunotherapy to BCG for patients who experience a relapse >1 year following BCG exposure. In a phase II trial of 1,106 patients with NMIBC recurrence >1 year following BCG exposure, Gallagher et al. were able to demonstrate that addition of interferon alpha to BCG leads to response rates similar to those observed among BCG-naïve patients.5
To conclude his presentation, Dr. Chen noted that:
- Low-grade recurrences following intravesical BCG therapy do not fulfill the criteria for BCG failure
- Treatment should be individualized according to the index tumor
- Quality of TURBT may affect recurrence rates
- BCG re-treatment is appropriate for late relapses
- A less aggressive approach may be tailored to fitness and age
At this point, Dr. Speiss made his argument in favor of surveillance and therapy de-escalation. He noted that when we talk about treatment de-escalation, we are mainly targeting those patients with low-risk disease, which is namely those patients with small volume, low-grade Ta disease.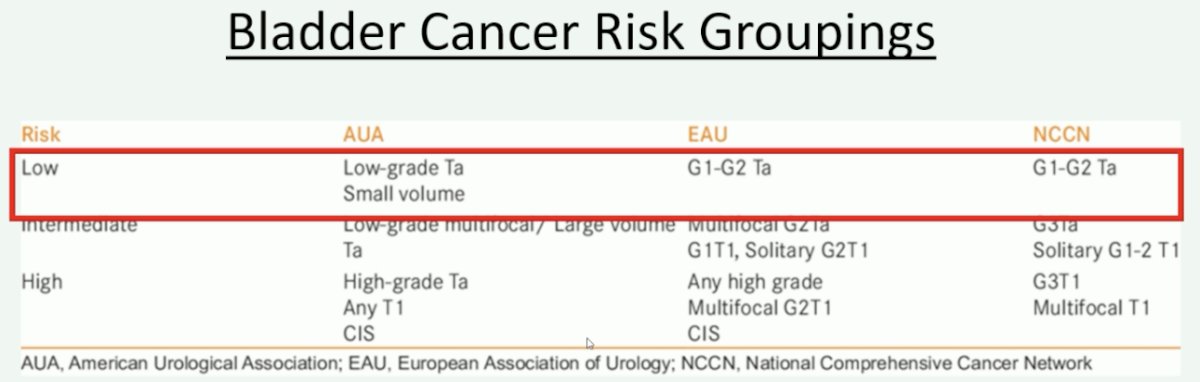
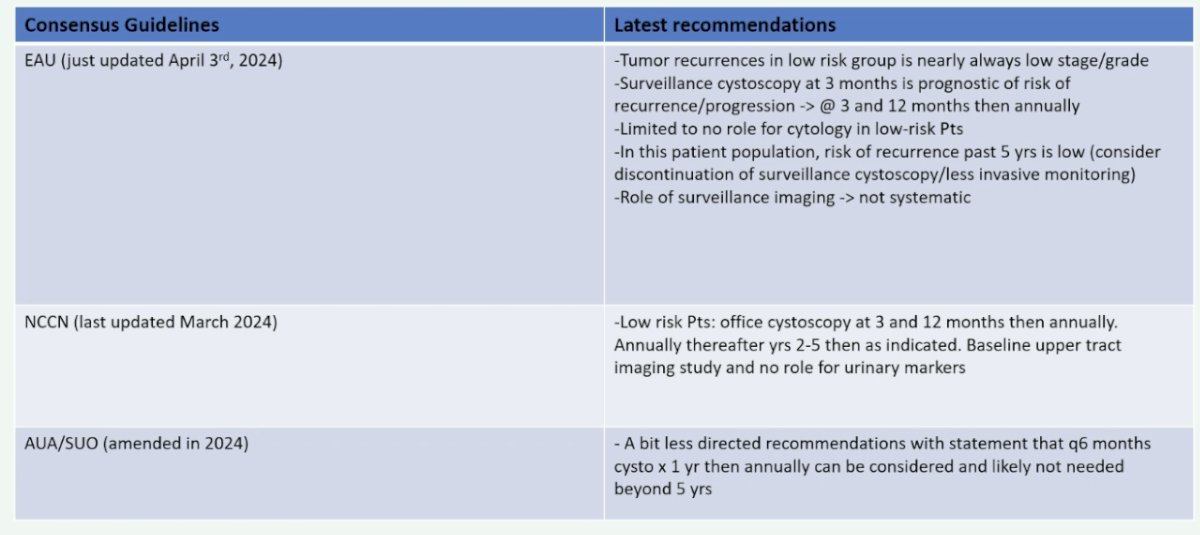
What do the guidelines tell us on this subject? The updated EAU guidelines, which were presented during this meeting (April 3rd, 2024), provide the best granularity on this topic:
- Tumor recurrences in low-risk group are nearly always low stage and grade
- Surveillance cystoscopy at 3 months is prognostic of risk of recurrence/progression. As such, perform at 3 and 12 months, and then annually thereafter.
- Limited to no role for cytology in low-risk patients
- In this patient population, the risk of recurrence past 5 years is low. As such, consider discontinuation of surveillance cystoscopy/less invasive monitoring.
- Role of surveillance imaging not systematic
What is the natural history of patients with low-risk NMIBC? A recent multicenter retrospective study of 390 patients meeting the 2015 NICE definition of low-risk NMIBC was published. At a median follow-up of 36 months, 29.2% of patients developed a recurrence. Notably, patients with Grade 2 tumors, as opposed to Grade 1 tumors, were more likely to develop a recurrence at 1 year (34% versus 27%). Only 1.8% of patients progressed to high-grade disease and an additional 1% experienced progression to muscle invasiveness. Notably, if a surveillance cut-off of 5 years were implemented, 96.5% of recurrences would be captured.6
We also need to consider the economic aspect of frequent surveillance and continued treatment. We know that bladder cancer is one of the most expensive malignancies, and this is directly related to the costs of frequent surveillance cystoscopies. Worrisomely, Medicare-related costs continue to increase annually, in part due to the over-performance/reliance on cystoscopies.
A recent population-based retrospective cohort study (using the SEER-linked Medicare database) evaluated the contemporary surveillance and treatment practices, cancer outcomes, and costs of care for low grade NMBC. This study included 13,054 patients aged 66 to 90 years from January 2004 to December 2014. The rates of surveillance cystoscopy increased over the study period (79.3% in 2004 to 81.5% in 2013), with patients having a median of 3 surveillance cystoscopies/year. Among patients with low grade Ta NMBC, only 52 (0.4%) experienced disease progression. Notably, the total median costs of surveillance testing and treatment in this cohort increased by 60% (from $34,792 in 2004 to $53,986 in 2013), with higher median expenditures among those with disease recurrence ($76,669) versus no disease recurrence ($53,909) across the study period.7
In 2021, the results of a non-blinded, two-arm randomized controlled trial of patients with low or low-intermediate risk NMIBC were performed randomizing patients to high versus low frequency surveillance following initial TURBT. The high frequency cohort was defined as surveillance every 3 months for two years then every 6 months for an additional two years, with annual follow-up thereafter. Conversely, in the low frequency group, surveillance was performed at 3 months, at 9 months, and then annually thereafter. This trial included 70 patients with disease recurrence observed in 14.3% and 20.8% of patients in the high versus low-frequency surveillance arms. Patients had similar patient-reported and quality-of-life outcomes across the two arms, and the out-of-pocket healthcare costs were $383.80 higher per patient annually in the high frequency cohort.8
The IBCG provides a contemporary update and recommendations for the diagnosis and management of low-grade NMIBC, using a comprehensive review of published trials, guidelines, meta-analyses, and reviews. The IBCG concluded that low-grade Ta bladder cancer poses minimal risk to patients in terms of risk of progression and disease-specific survival (0.5 – 2.8%), as well as a low risk of developing UTUC (0.6–8%), often low-grade as well. Low-grade appearing bladder recurrences can be surveilled if asymptomatic or managed by office cystoscopy and fulguration. Notably, surveillance cystoscopy can be discontinued after 5 years.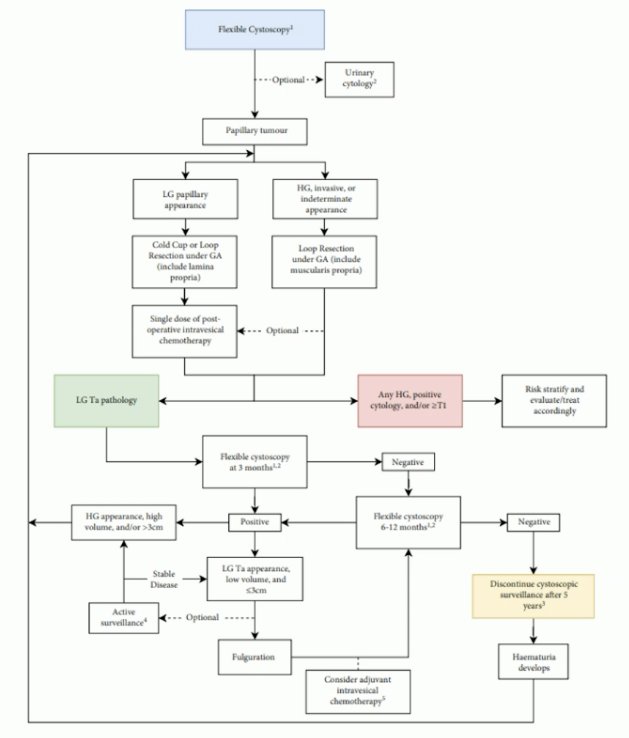
Presented by:
- Professor Marek Babjuk, MD, PhD, Department of Urology, 2nd Faculty of Medicine, Hospital Motol, Prague, Czech Republic
- Kenneth Chen, MBBS, MCI, MRCS (Edin), FRCS (Glasg), FAMS, Consultant, Singapore General Hospital, Singapore
- Philippe E. Spiess, MD, MS, Professor, Department of Genitourinary Oncology Moffitt Cancer Center Tampa, Florida
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:- Mmeje CO, Guo CC, Shah JB, et al. Papillary Recurrence of Bladder Cancer at First Evaluation after Induction Bacillus Calmette-Guérin Therapy: Implication for Clinical Trial Design. Eur Urol. 2016;70(5): 778-785.
- Garg T, McMullen CK, Leo MC, et al. Predicting risk of multiple levels of recurrence and progression after initial diagnosis of nonmuscle-invasive bladder cancer in a multisite, community-based cohort. Cancer. 2021;127(4): 520-527.
- Lindgren MS, Hansen E, Azawi N, et al. DaBlaCa-13 Study: Oncological Outcome of Short-Term, Intensive Chemoresection With Mitomycin in Nonmuscle Invasive Bladder Cancer: Primary Outcome of a Randomized Controlled Trial. J Clin Oncol. 2023;41(2): 206-211.
- Li R, Metcalfe MJ, Tabayoyong WB, et al. Using Grade of Recurrent Tumor to Guide Further Therapy While on Bacillus Calmette-Guerin: Low-grade Recurrences Are not Benign. Eur Urol Oncol. 2019;2(3): 286-293.
- Gallagher BL, Joudi FN, Maymi JL, O’Donnell MA. Impact of previous bacille Calmette-Guérin failure pattern on subsequent response to bacillus Calmette-Guérin plus interferon intravesical therapy. Urology. 2008;71(2): 297-301.
- Jaffer A, Lee M, Khalil O, et al. The natural history of low-risk non-muscle-invasive bladder cancer: a collaborative multi-centre study. Int Urol Nephrol. 2022;54(9): 2175-2180.
- Bree KK, Shan Y, Hensley PJ, et al. Management, Surveillance Patterns, and Costs Associated With Low-Grade Papillary Stage Ta Non-Muscle-Invasive Bladder Cancer Among Older Adults, 2004-2013. JAMA Netw Open. 2022;5(3): e223050.
- Reyes RM, Rios E, Barney S, et al. A Randomized Feasibility Trial Comparing Surveillance Regimens for Patients with Low and Low-Intermediate Risk Non-Muscle Invasive Bladder Cancer. Bladder cancer. 2021;7(3): 285-295.


