(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Professors Andrea Necchi, Peter Black, and Jørgen Bjerggaard participated in a rapid-fire debate discussing whether we can use circulating tumor DNA (ctDNA) and/or urine tumor DNA (utDNA) in patients with muscle-invasive bladder cancer (MIBC) following radical cystectomy to guide treatment decision on adjuvant therapy.
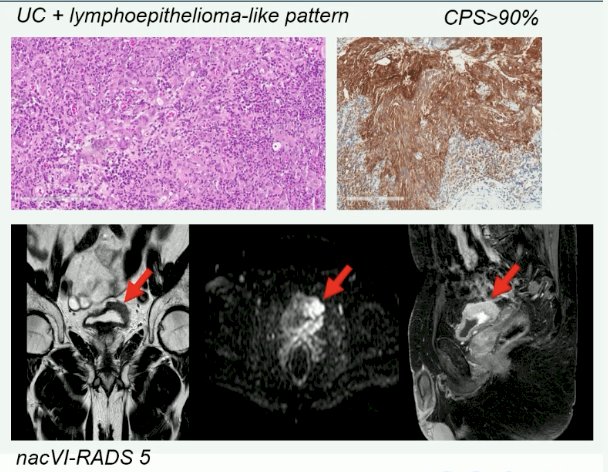
Professor Necchi presented a case of a 52-year-old female marathon athlete with prior excellent health. Patient had experienced 9 months of intermittent hematuria, which she had attributed to ‘runners hematuria’. Evaluation with a cystoscopy and imaging was consistent with a sessile mass in the bladder wall. She was diagnosed with cT3N0M0 MIBC. Her renal function was adequate (GFR of 67 mL/min), and she denied neuropathy, hearing loss, or congestive heart failure. She received 4 cycles of neoadjuvant cisplatin + gemcitabine followed by a radical cystectomy + neobladder formation. On final pathology, she had evidence of pT3b disease with all 32 lymph nodes resected negative for nodal involvement.
Professor Peter Black proceeded to argue his viewpoint that ctDNA and utDNA are not ready for prime-time use to guide MIBC management. At the current time, we have level 1 evidence for both neoadjuvant chemotherapy and adjuvant immunotherapy; however, we have no definitive evidence for the use of ctDNA or utDNA in either setting. As such, we cannot ‘give up’ the disease-free and overall survival benefits of these proven treatments based on unvalidated biomarkers.
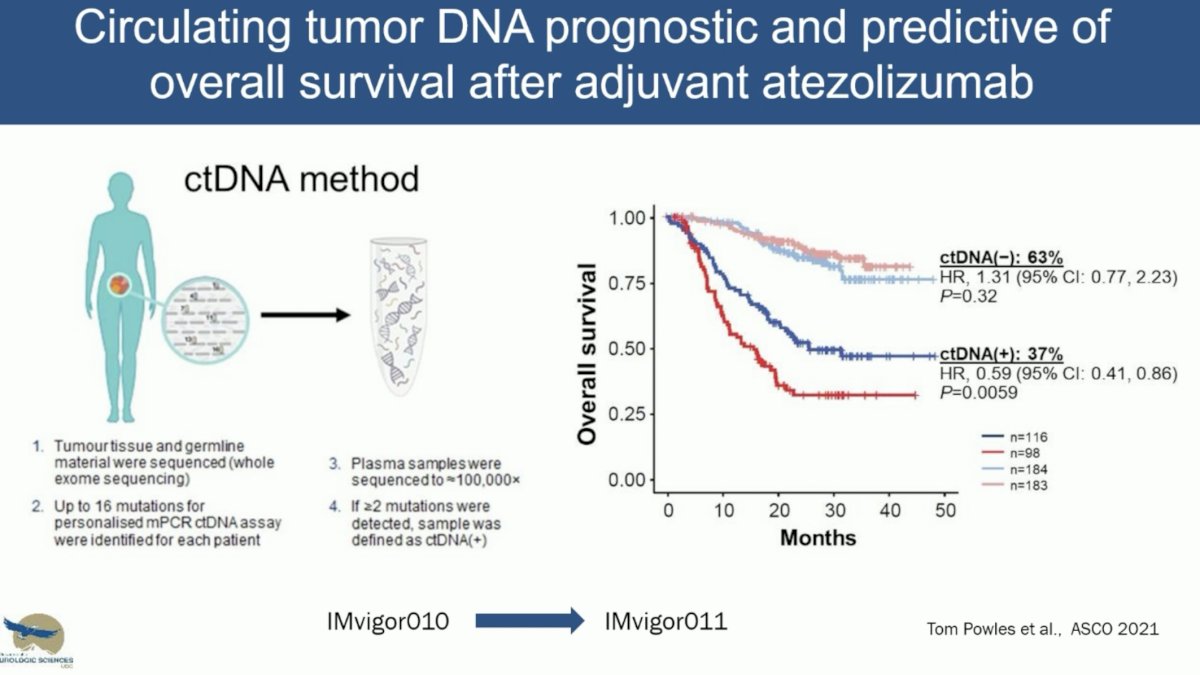
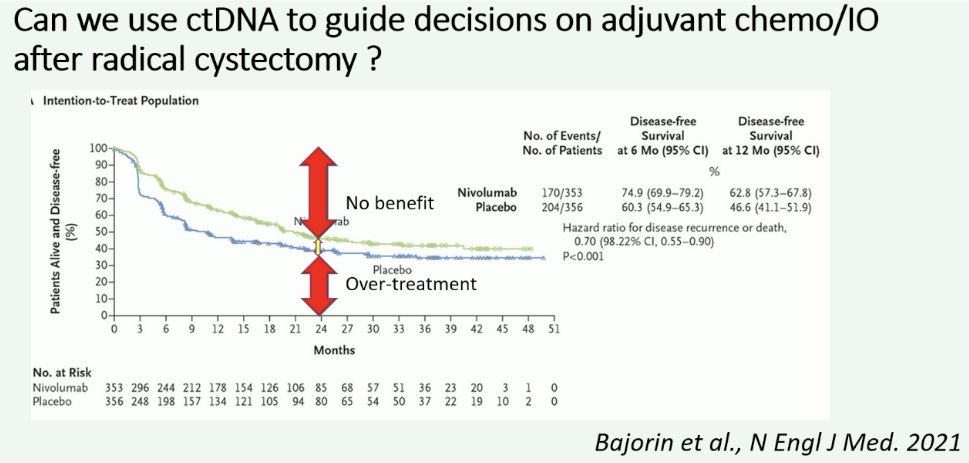
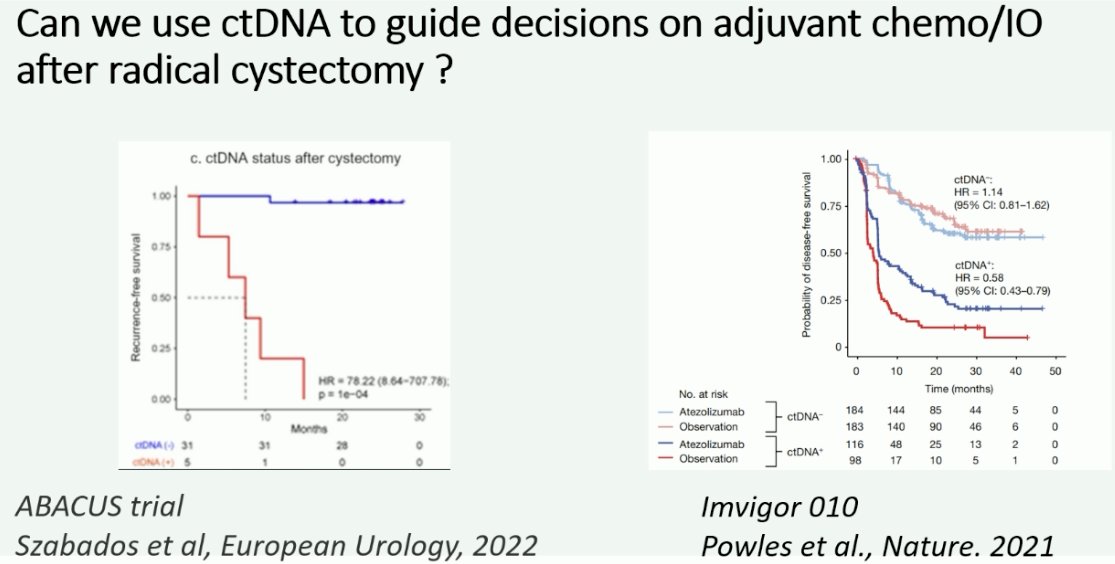
What is the evidence for ctDNA use in the adjuvant setting following a radical cystectomy? To date, we have evidence of a disease-free survival benefit with the use of nivolumab (CheckMate-274)1 and pembrolizumab (AMBASSADOR), with overall survival data from CheckMate-274 presented at EAU 2024 and demonstrating an overall survival benefit for nivolumab in this setting (HR 0.76, 95% CI: 0.61 – 0.96). A third trial of adjuvant immunotherapy in this setting is IMvigor-010, which failed to demonstrate a disease-free survival benefit for atezolizumab. However, ad hoc analysis of this trial demonstrated that ctDNA was both prognostic and predictive of overall survival with adjuvant atezolizumab. In the observation arm, ctDNA positivity versus negativity was associated with shorter overall survivals (HR: 6.3, 95% CI: 4.3–9.3). The ctDNA positivity identified patients with an overall survival benefit favoring atezolizumab versus observation (HR: 0.59, 95% CI: 0.42–0.83).2
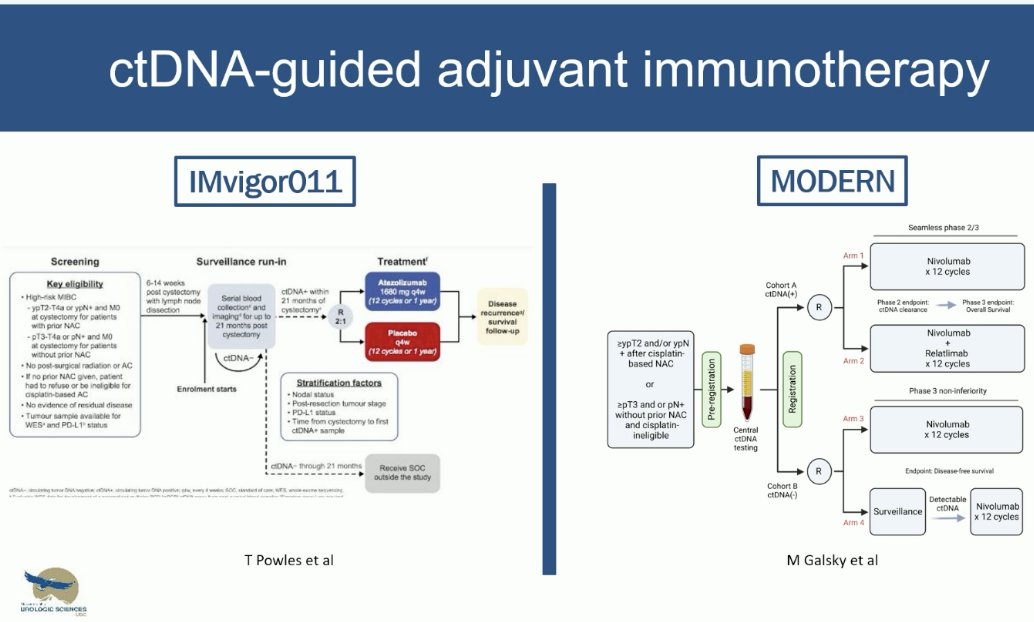
Prospective trials in this space that are evaluating ctDNA-guided adjuvant immunotherapy include IMvigor011 and MODERN. The initial results of IMvigor011 were also presented at EAU 2024, and Dr. Black noted that they demonstrate promising outcomes for the ctDNA- patients.
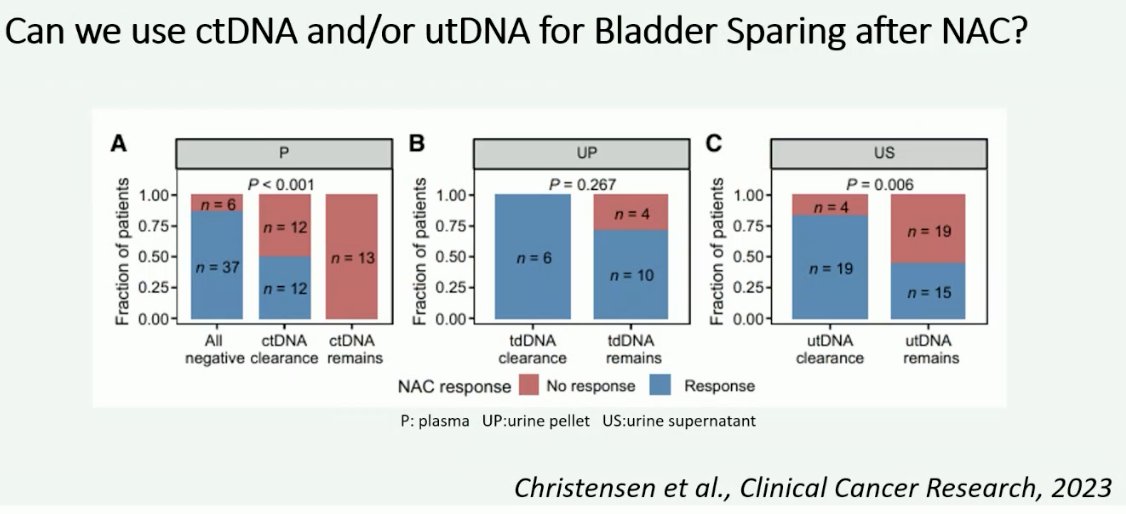
What about utDNA to guide bladder preservation? This modality may be of ultimate importance for patients who are being considered for bladder-preserving strategies, whereby patients who have no evidence of clinically residual disease following neoadjuvant therapy, including a negative utDNA, may be considered for bladder-sparing approaches. Examples of bladder-sparing trials include RETAIN, HOOSIER, A031701, and RETAIN2. However, to date, none of these trials have reported data on ctDNA or utDNA.

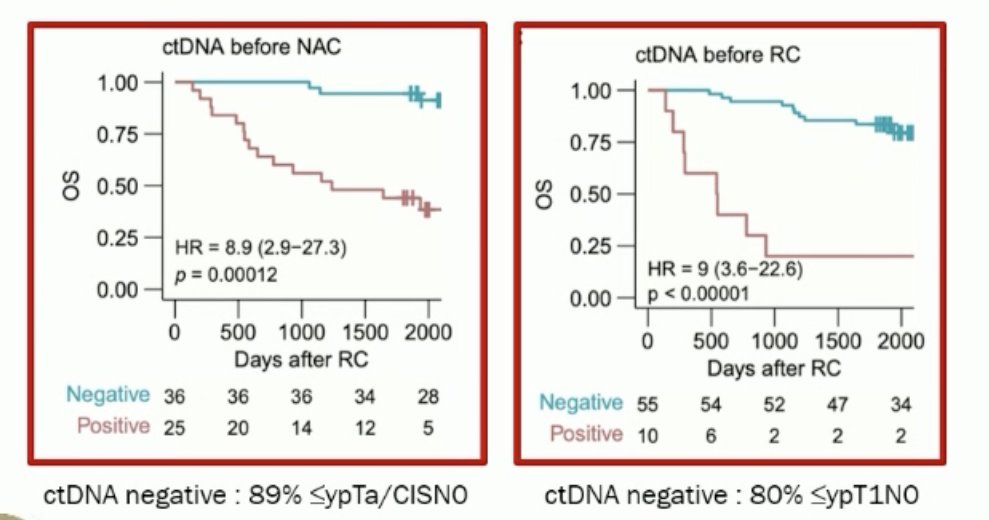
There is evidence, however, for the use of ctDNA/utDNA to predict outcomes following neoadjuvant chemotherapy in patients with MIBC undergoing a radical cystectomy. In 2023, Lindskrog et al. demonstrated that ctDNA following radical cystectomy identified metastatic relapse with a sensitivity of 94% and specificity of 98%. ctDNA dynamics during neoadjuvant chemotherapy were independently associated with patient outcomes when adjusted for pathologic downstaging (HR: 4.7; p=0.029).3 He however argued that ctDNA had a negative predictive value of only 89% in the pre-neoadjuvant setting and 80% prior to radical cystectomy for predicting ≤ypTa/CISN0 disease. While these values appear to be high, ultimately, we will require values closer to 100% when considering bladder preservation for these patients.
Theoretical limitations to ctDNA/utDNA use for bladder preservation include concerns about their sensitivity in the limited disease setting. Radical cystectomy specimens frequently show microscopic foci of residual high grade urothelial carcinoma in the lamina propria, muscle, or fat. He noted that there is no reason to suspect exposure of tumor to urine for utDNA. Conversely, would there be enough tumor burden to be systemically measured via ctDNA.
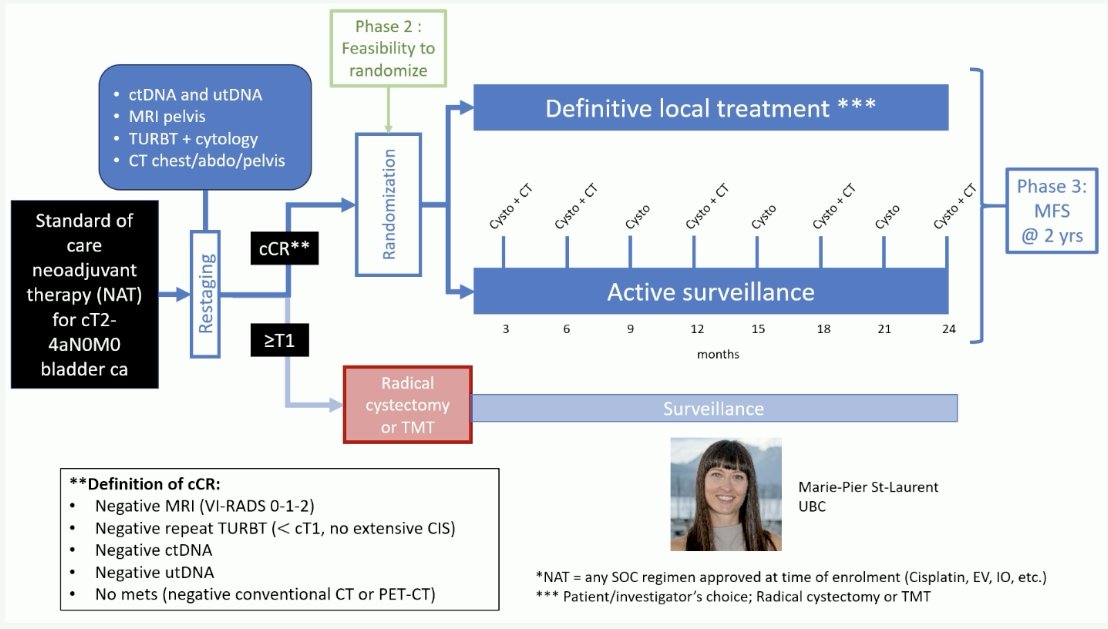
Can combined use of all available modalities (cytology, CT, cystoscopy/TURBT, ctDNA, utDNA, and MRI) enhance the accuracy of clinical re-staging after neoadjuvant systemic therapy to make bladder preservation safer? The combination of these tools is being assessed in a phase 2 bladder preservation trial, whereby patients with cT2-4aN0M0 disease with a complete clinical response, defined by a negative MRI (VI-RADS 0–2), negative repeat TURBT (<cT1, no extensive CIS), negative ctDNA/utDNA, and no metastases on CT are being randomized to definitive local treatment versus active surveillance.
Next, Professor Jensen made the case for the use of ctDNA/utDNA to guide the management of MIBC patients. While we have seen overall survival benefits with the use of adjuvant nivolumab from the CheckMate-274 trial, Professor Jensen noted that the differences are not that clinically meaningful, and there is a significant proportion of patients that do not benefit from adjuvant therapy, and, as such, were overtreated. Accordingly, we need to do better to personalize treatment decisions for these high-risk patients.
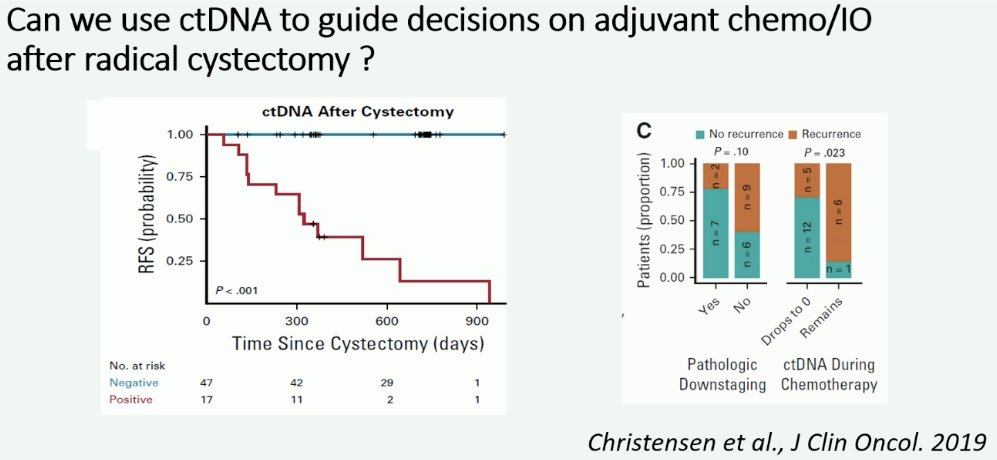
In 2019, Dr. Jensen’s group published a report of 68 patients with localized, advanced bladder cancer that demonstrated that the presence of ctDNA was highly prognostic at diagnosis before chemotherapy (HR: 29.1; p=0.001). After cystectomy, ctDNA analysis correctly identified all patients with metastatic relapse during disease monitoring (100% sensitivity, 98% specificity), whereby none of the ctDNA- patients recurred during follow-up, compared to all ctDNA+ patients recurring.4 He argued that it may even be considered ‘unethical’ to randomize ctDNA- patients to adjuvant immunotherapy versus observation in clinical trials, given that none of the ctDNA- patients recurred in this trial.
Further support for the use of ctDNA to guide decision on adjuvant immunotherapy following radical cystectomy comes from the ABACUS trial (neoadjuvant atezolizumab), whereby ctDNA was shown to be strongly prognostic for recurrence-free survival outcomes following cystectomy. Additionally, the aforementioned ad hoc analysis of IMvigor-010 has demonstrated that ctDNA is a predictive marker of treatment response to adjuvant atezolizumab following radical cystectomy.
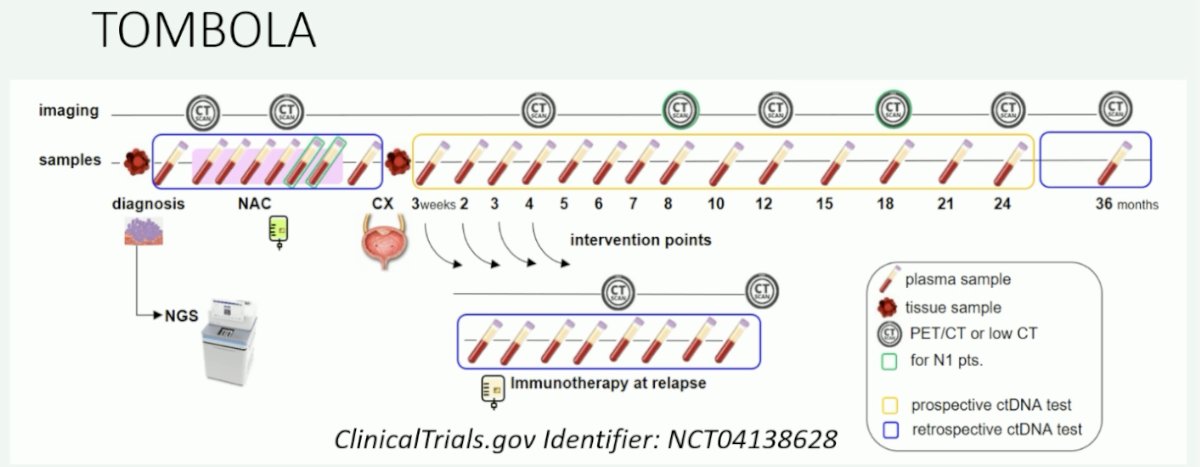
The TOMBOLA trial (NCT04138628) is evaluating the use of serial ctDNA in patients with MIBC receiving neoadjuvant chemotherapy followed by a radical cystectomy, with patients recommended for immunotherapy only at relapse, defined by a positive ctDNA test result.
With regards to utDNA, Professor Jensen noted that the evidence is weaker to date. utDNA may eventually hold more value for the evaluation of persistent, local residual disease. For the time being though, ctDNA holds more value for guiding treatment decision making.
Presented by:
- Professor Andrea Necchi, MD, Director of GU Medical Oncology, San Raffaele Hospital and Scientific Institute, Milan, Italy
- Professor Peter Black, MD, Department of Urologic Sciences, Vancouver Prostate Center, University of British Columbia, BC, Canada
- Professor Jørgen Bjerggaard Jensen, MD, DMSc, Chair, Department of Urology, Aarhus University, Aarhus, Denmark
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22): 2102-2114.
- Powles T, Assaf ZJ, Degaonkar V, et al. Updated Overall Survival by Circulating Tumor DNA Status from the Phase 3 IMvigor010 Trial: Adjuvant Atezolizumab Versus Observation in Muscle-invasive Urothelial Carcinoma. Eur Urol. 2024;85(2): 114-122.
- Lindskrog SV, Birkenkamp-Demtroder K, Nordentoft I, et al. Circulating Tumor DNA Analysis in Advanced Urothelial Carcinoma: Insights from Biological Analysis and Extended Clinical Follow-up. Clin Cancer Res. 2023;29(23): 4797-4807.
- Christensen E, Birkekamp-Demtroder K, Sethi H, et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J Clin Oncol. 2019;37(18): 1547-1557.
- Szabados B, Kockx M, Assaf ZJ, et al. Final Results of Neoadjuvant Atezolizumab in Cisplatin-ineligible Patients with Muscle-invasive Urothelial Cancer of the Bladder. Eur Urol. 2022;82(2): 212-222.


