(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a session on non-muscle invasive bladder cancer, and a presentation by Dr. James Catto discussing the trial design of SunRISe-3, assessing TAR-200 plus cetrelimab or TAR-200 versus intravesical BCG in patients with BCG-naive high-risk non–muscle-invasive bladder cancer. Standard of care for patients with high-risk non–muscle-invasive bladder cancer is transurethral resection of the bladder tumor (TURBT; biopsy only for carcinoma in situ) followed by intravesical BGG. However, BCG is associated with toxicities and there are supply shortages and a lack of durable response in a significant proportion of patients. TAR-200 is an intravesical drug delivery system designed for sustained local gemcitabine release within the bladder:
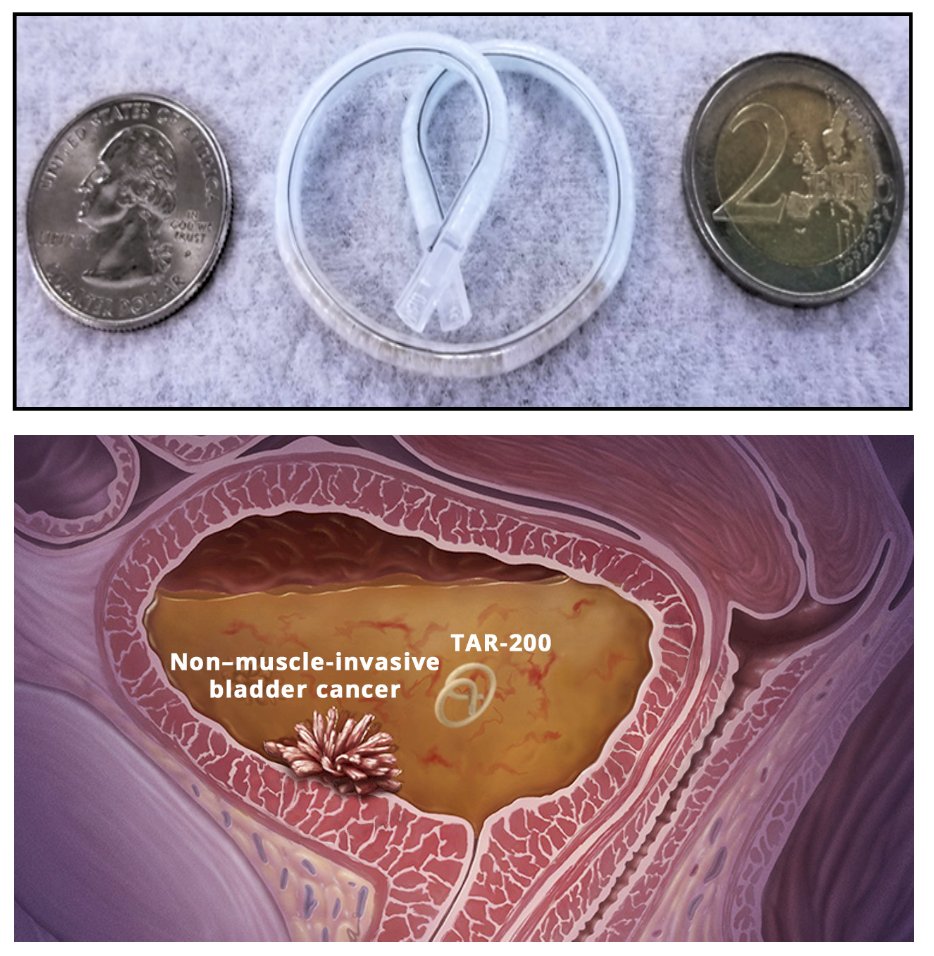
Preliminary data from SunRISe-1 support further investigation of TAR-200 with or without cetrelimab in patients with BCG-naïve high-risk non–muscle-invasive bladder cancer. SunRISe-3 is an open-label, multicenter, randomized phase 3 study designed to assess the efficacy and safety of TAR-200 + systemic cetrelimab or TAR-200 alone versus BCG in patients with BCG-naïve high-risk non–muscle-invasive bladder cancer:
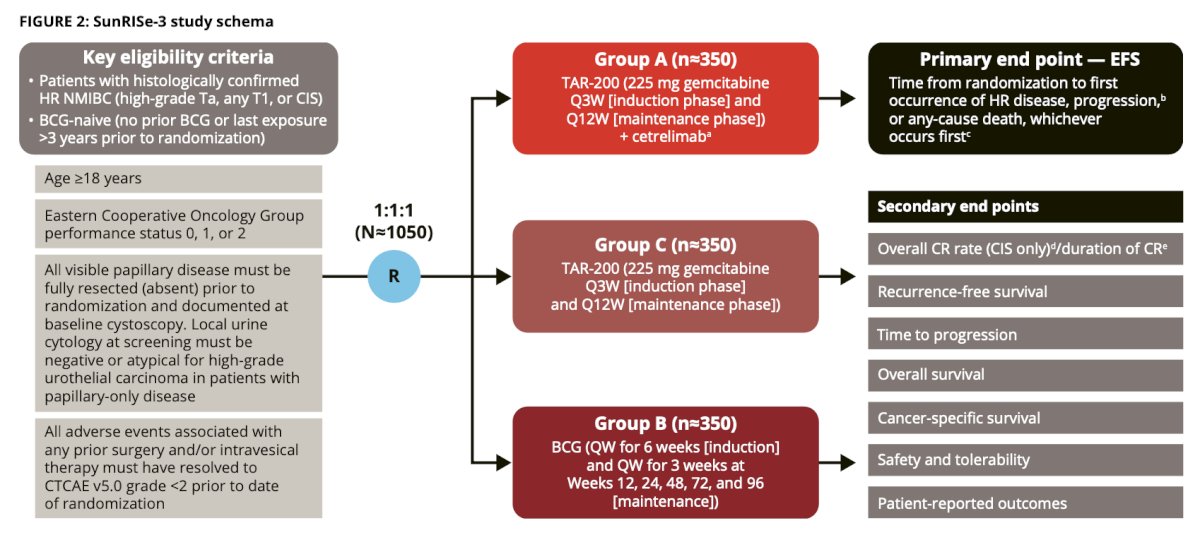
Eligible BCG-naïve high-risk non–muscle-invasive bladder cancer patients (n = 1,050) will be randomized 1:1:1 to receive TAR-200 + cetrelimab, TAR-200, or BCG. In cohort B, BCG will be supplied for the duration of the patient’s treatment and dosing may continue up to an additional third year at the investigator’s discretion. The primary endpoint is event free survival and secondary endpoints including efficacy, safety, and patient-reported outcomes. At screening and during treatment phases (treatment phase is the first 2 years), assessment will be cystoscopy and urine cytology (weeks 12, 24, 36, and 48), and TURBT/bladder biopsy is mandatory at week 24 for CIS, and anytime as clinically indicated for other cases. CT/MR urogram will performed every 24 weeks for CIS patients and every 48 weeks for papillary patients during the treatment phase. Recruitment for SunRISe-3 is currently ongoing with recruitment planned at 248 sites. SunRISe-3 is currently recruiting in Argentina, Australia, Belgium, Brazil, Canada, China, Czechia, France, Germany, India, Italy, Poland, South Korea, Spain, Taiwan, and the United States:
The SunRISe-3 study opened for enrollment in March 2023, with 153 patients in screening and 577 patients randomized as of January 26, 2024.
Presented by: James W.F. Catto, University of Sheffield, Sheffield, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024


