(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a session on non-muscle invasive bladder cancer, and a presentation by Dr. Thorsten Ecke discussing validation of FGFR screening by Uromonitor® and Therascreen in FFPE tissue in bladder cancer in the context of the Bladder BRIDGister clinical pathological real-world registry study. Bladder cancer patients with FGFR alterations may benefit from targeted FGFR therapies, however, conventional risk stratification and FGFR testing is based on tissue diagnostics at initial diagnosis. As such, FGFR mutation screening will become more important in therapeutic decisions for bladder cancer patients, and there is a high need for optimized screening methods. The aim of the present study presented at EAU 2024 was to prospectively evaluate the FGFR mutation detection in urine and tissue samples from patients with suspected bladder cancer undergoing TURBT as part of the “Bladder BRIDGister” Real World Experience study.
There were 398 paraffin-embedded tissue samples from TURBT samples prospectively collected and analyzed. These specimens were analyzed with uromonitor® (Porto, Portugal) for FGFR mutations R248C, S249C, G370C, and Y373C for 197 samples (Charge 1) and R248C, S249C for 200 samples (Charge 2). RNA extraction was performed with commercial kits and the Therascreen FGFR IVD-Kit (Hilden, Germany) for 309 samples detecting R248C, S249C, and Y373C.
Both tests were in accordance with 266 of 309 measurements (86%; kappa 0.66; p<0.001), and 199 wildtype and 67 mutations have been detected. Uromonitor detected 28 mutations, whereas Therascreen detected wildtype. Therascreen detected 15 mutations, whereas Uromonitor detected wildtype. The following figure demonstrates the frequency of FGFR alterations when being tested by tissue based IVD test (Therascreen) versus urine based IVD test (Uromonitor):
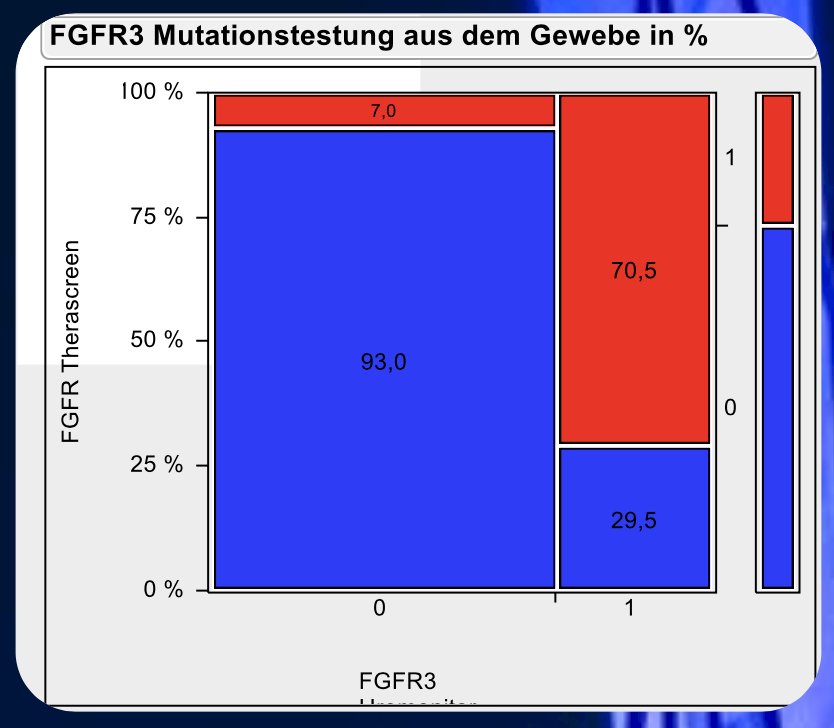
The following figure highlights the frequency of FGFR alterations tested by the tissue based IVD test (Therascreen) stratified by Ta and T1 non-muscle invasive bladder cancer:

There was a statistically significant correlation between FGFR3 mutation and FGFR3 mRNA overexpression (p < 0.001): Therascreen (r = 0.59) and Uromonitor (r = 0.51).
Dr. Ecke concluded his presentation by discussing validation of FGFR screening in the Bladder BRIDGister real-world registry study with the following conclusions:
- This molecular registry reflects real frequency of urinary bladder cancer
- FGFR3 mutations are rare in early stages
- FGFR3 mutations S249C and R248C are most frequent detected
- Uromonitor and Therascreen tissue tests are comparable
Future directions include a prospective assessment of FGFR mutation detection to assess the efficacy of standard treatment as part of the “Bladder BRDGister” trial, and assessment of prognostic and predictive value of FGFR alteration detection in molecularly stratified clinical trials introducing FGFR targeted treatment in early and advanced stages of bladder cancer.
Presented by: Thorsten Ecke, Helios Klinikum, Bad Saarow, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024


