(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a session on non-muscle invasive bladder cancer, and a presentation by Dr. Andrea Necchi discussing results from SunRISe-1 in patients with BCG-unresponsive high-risk non–muscle-invasive bladder cancer receiving TAR-200 monotherapy. Standard of care for BCG-unresponsive high-risk non-muscle invasive bladder cancer is a radical cystectomy, however radical cystectomy is associated with significant rates of morbidity and mortality, as well as an impact on quality of life. Patients with BCG-unresponsive high-risk non–muscle-invasive bladder cancer are at high risk of disease progression with limited treatment options. The 12-month complete response rate for pembrolizumab is 19%, for atezolizumab is 15%, and for nadofaragene firadenovec is 23%. TAR-200, an intravesical drug delivery system, provides sustained release of gemcitabine in the bladder over many days:

SunRISe-1 (NCT04640623) is an ongoing, randomized, phase 2b study assessing the efficacy and safety of TAR-200 + cetrelimab (anti-PD1) (Cohort 1), TAR-200 alone (Cohort 2), or cetrelimab alone (Cohort 3) in patients with BCG-unresponsive high-risk non–muscle-invasive bladder cancer ineligible for or refusing radical cystectomy. As of Amendment 4, patients with papillary disease only will be enrolled in Cohort 4 and treated with TAR-200 alone. At the EAU 2024 annual meeting, Dr. Necchi reported results from Cohort 2.
Institutional review board approval and informed consent were obtained for this study. Eligible patients aged ≥18 years had histologically confirmed carcinoma in situ (CIS) ± papillary disease (high-grade Ta, any T1), Eastern Cooperative Oncology Group performance status of 0-2, and persistent or recurrent high-risk non–muscle-invasive bladder cancer with last dose of BCG ≤12 months prior to CIS diagnosis. TAR-200 was dosed every 3 weeks through week 24, then every 12 weeks until week 96. Response assessments were by cystoscopy and centrally assessed urine cytology, CT/MRI, and bladder biopsy (at weeks 24, 48, and as clinically indicated). The study design for SunRISe-1 is as follows:
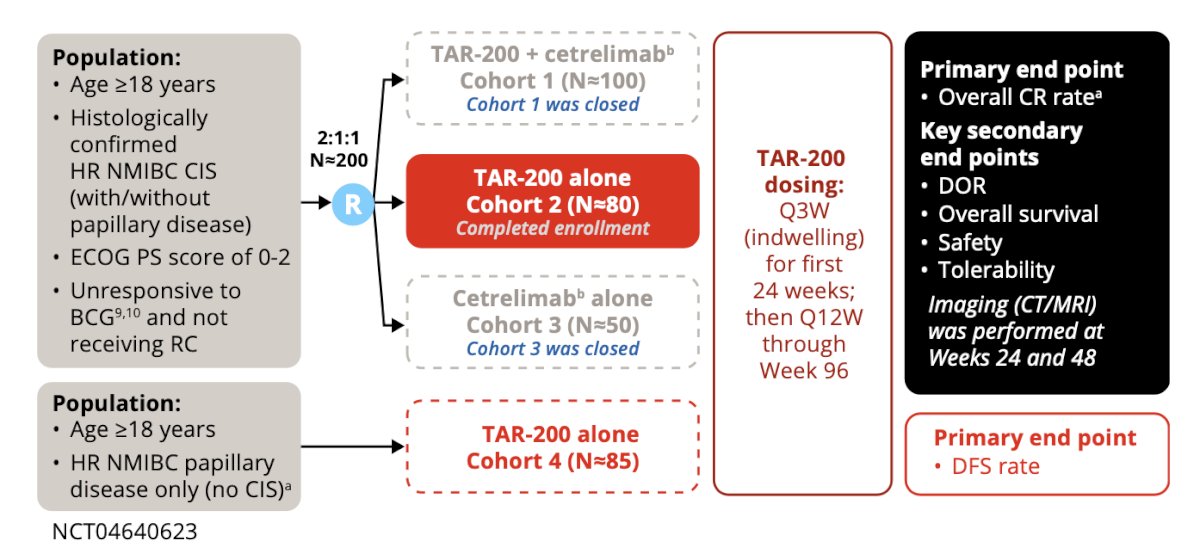
The primary endpoint was overall complete response rate. Secondary endpoints included duration of response, overall survival, safety, and tolerability.
As of the August 24, 2023 data cutoff, 54 patients (median age, 71 years; range, 40-85; 33% with concurrent papillary disease) received TAR-200 monotherapy. The baseline demographics are as follows:
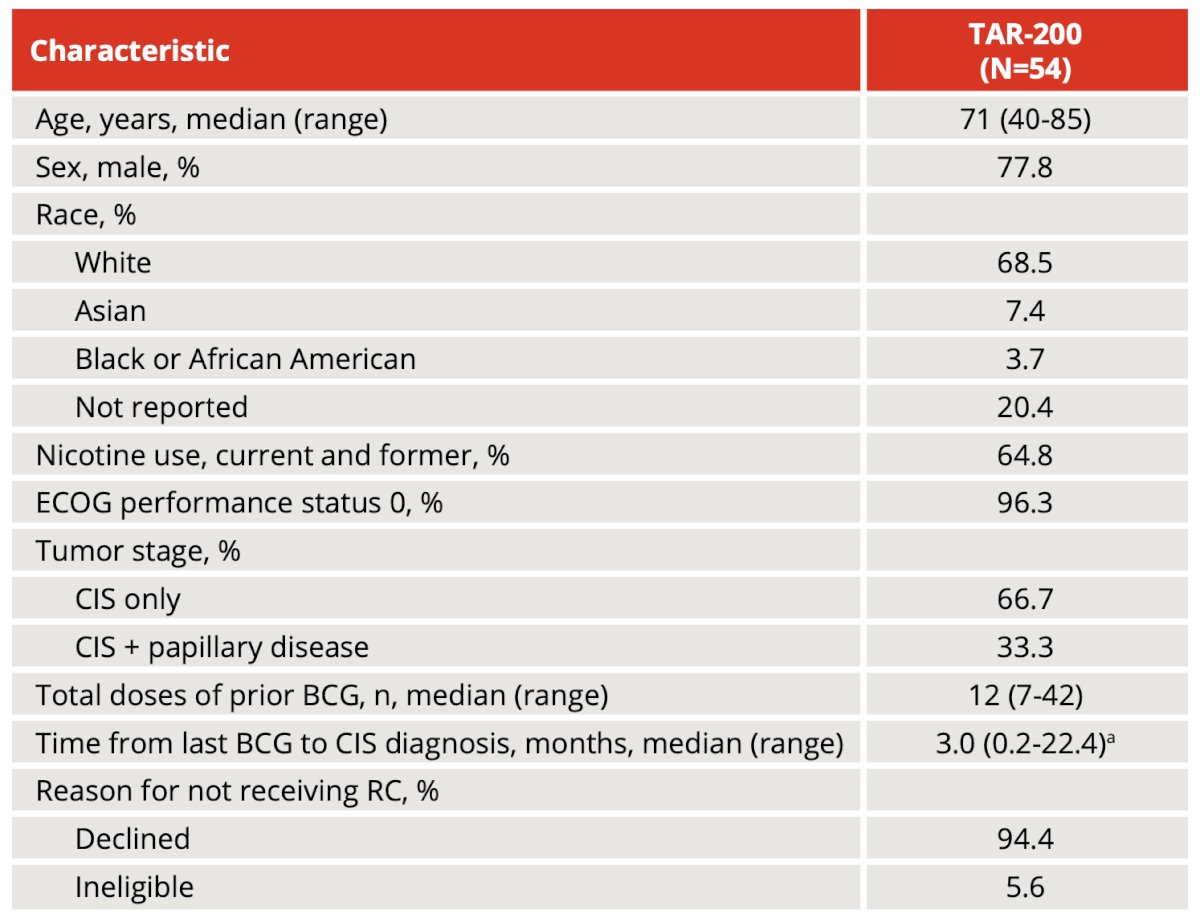
Overall, there were 30 patients available for efficacy evaluation. Centrally confirmed complete response by urine cytology and/or biopsy was achieved in 23/30 patients (77%; 95% CI, 58-90). Complete response rate by investigator assessment was comparable with central results:

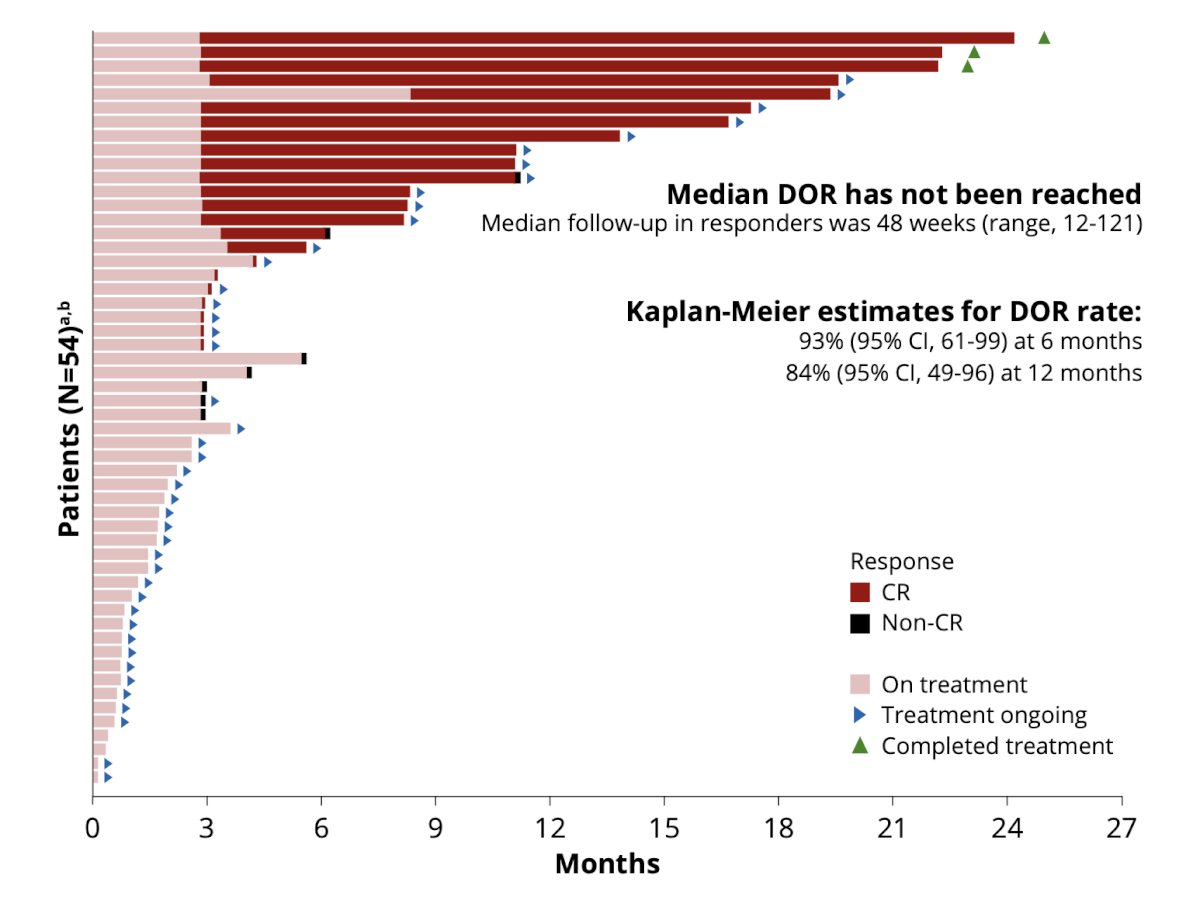
Median duration of response was not reached (median follow-up in responders, 48 weeks; range, 12-121); 21/23 responders remained in complete response with 10/11 and 6/6 having response ≥6 months and ≥12 months from complete response, respectively. None of the patients achieving a complete response have undergone radical cystectomy:
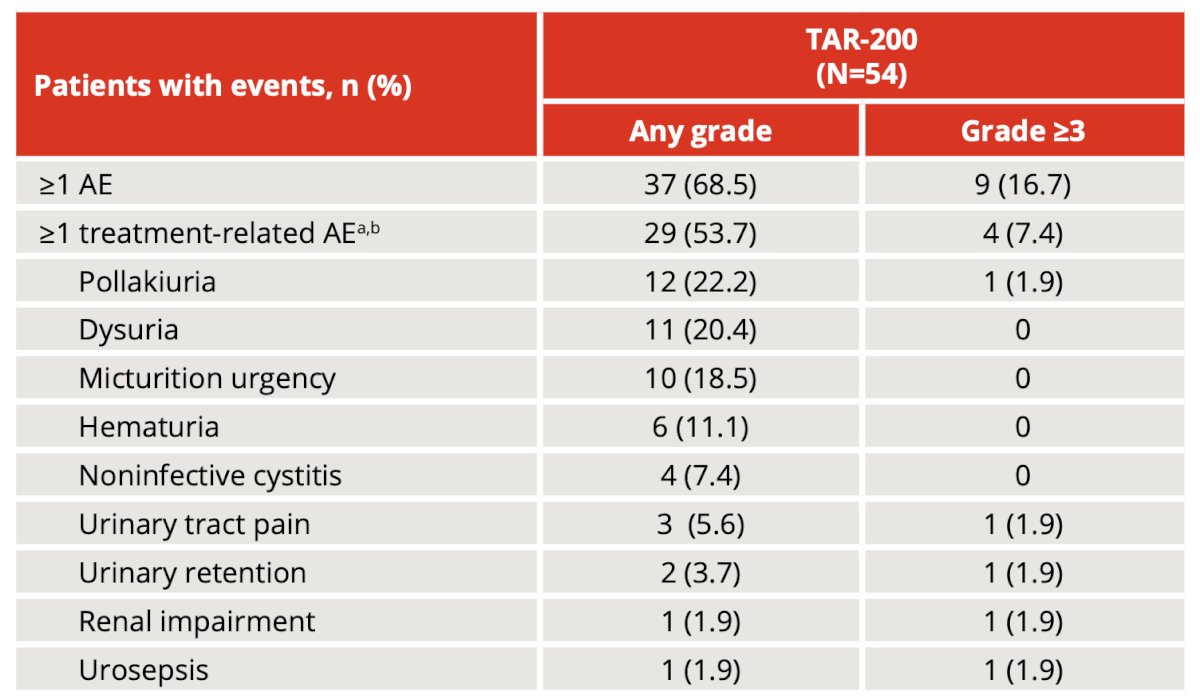
Overall, most adverse events in the TAR-200 cohort were grade 1 or 2, and 29 patients (54%) had treatment-related adverse events, most commonly (≥10%) pollakiuria, dysuria, micturition urgency, and hematuria. There were 4 patients (7%) that had grade ≥3 treatment-related adverse events, 1 (2%) had a serious treatment-related adverse event, and 2 (4%) had treatment-related adverse events leading to discontinuation. No deaths were reported:
Dr. Necchi concluded his presentation by discussing results from SunRISe-1 in patients with BCG-unresponsive high-risk non–muscle-invasive bladder cancer receiving TAR-200 monotherapy with the following conclusions:
- Overall, the centrally assessed complete response rate with TAR-200 monotherapy was 76.7% in BCG-unresponsive high-risk non-muscle invasive bladder cancer, with concordance with the investigator assessments
- TAR-200 provides a sustained and durable response with 91% of responses ongoing at clinical cutoff, and 6 patients having a duration of response of >=12 months
- TAR-200 was well tolerated, with mainly low-grade (1 or 2) adverse events related to the urinary tract system that were manageable with symptomatic treatment. TAR-200-related serious adverse events, grade >= 3 adverse events, and discontinuations were infrequent
Presented by: Andrea Necchi, MD, IRCCS San Raffaele Hospital, Vita-Salute San Raffaele University, Milan, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024


