(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a session on non-muscle invasive bladder cancer, and a presentation by Dr. Antoni Vilaseca discussing the first safety and efficacy results of the TAR-210 erdafitinib intravesical delivery system in patients with non–muscle-invasive bladder cancer with select FGFR alterations. Treatment options are limited in non–muscle-invasive bladder cancer that recurs after intravesical chemotherapy or BCG. FGFR alterations are prevalent in ~50% to 80% of non-muscle invasive bladder cancer and may function as oncogenic drivers. Erdafitinib is an oral selective pan-FRFR3 tyrosine kinase inhibitor approved in the US to treat FGFR3-alterated locally advanced or metastatic urothelial carcinoma after progression on or after at least one prior systemic treatment, with additional approvals across geographies. TAR-210 is a novel intravesical drug delivery system designed to provide local, continuous release of erdafitinib within the bladder while limiting systemic toxicities. This open-label, multicenter phase 1 study (NCT05316155) evaluated the safety, pharmacokinetics, and efficacy of TAR-210 in patients with non–muscle-invasive bladder cancer whose tumors harbor select FGFR alterations.
Institutional review board approval and informed consent were obtained for this study. FGFR alterations were identified in tumor tissue or urine cell-free DNA. Cohort 1 patients had recurrent, BCG-experienced high-risk non–muscle-invasive bladder cancer (high-grade Ta/T1; papillary only) and refused or were ineligible for radical cystectomy. Cohort 3 patients had recurrent, intermediate-risk non–muscle-invasive bladder cancer (Ta/T1) with a history of only low-grade papillary disease. Before treatment, Cohort 1 patients must have all visible disease resected, whereas Cohort 3 required the presence of visible tumors. TAR-210 systems with two different erdafitinib release rates were evaluated. Response was assessed every 3 months with continued treatment for up to 1 year if recurrence-free (Cohort 1) or in complete response (Cohort 3). The trial design for TAR-210 is as follows:

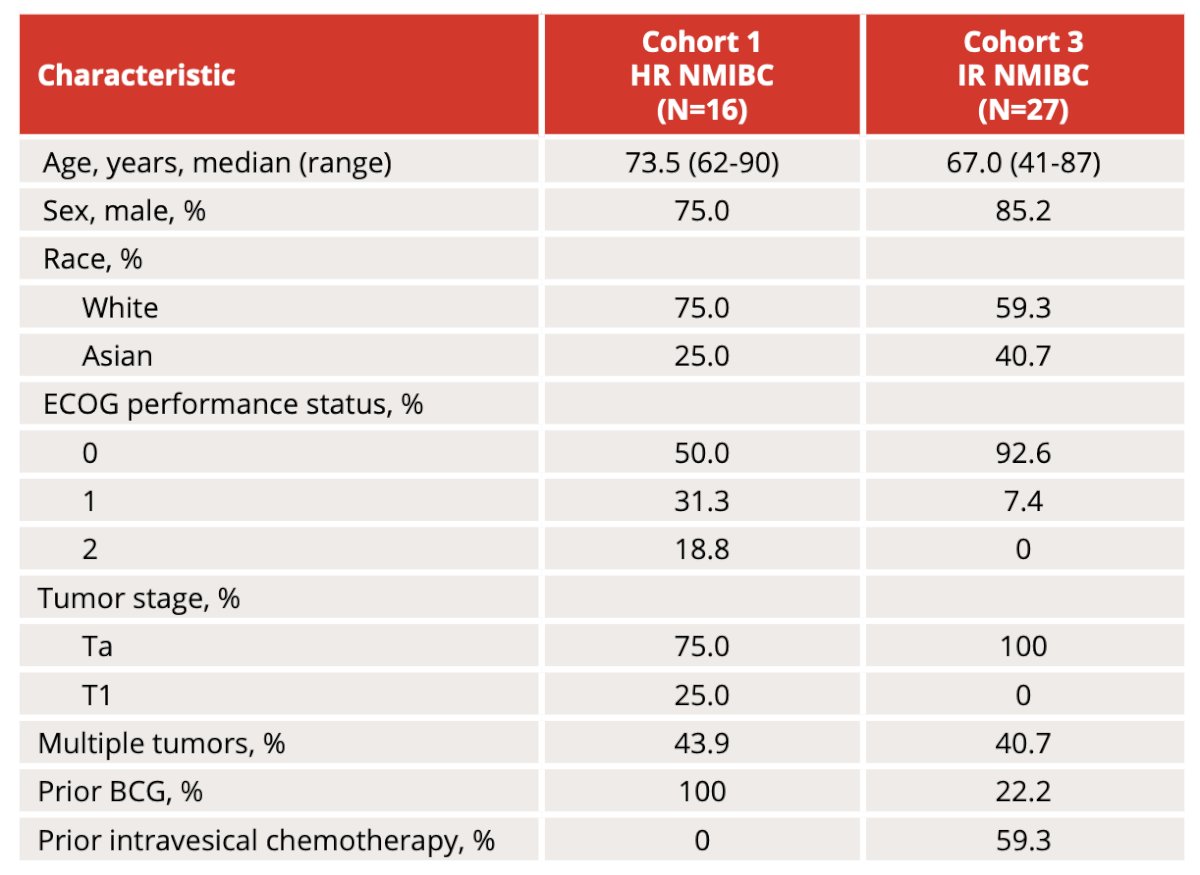
As of August 29, 2023, 16 patients in Cohort 1 and 27 patients in Cohort 3 have been treated with all tumors in both cohorts being recurrent. The Table 1 baseline characteristics are as follows:
In cohort 1, 11 patients had response assessment and 9 were recurrence free (recurrence free rate of 82%):
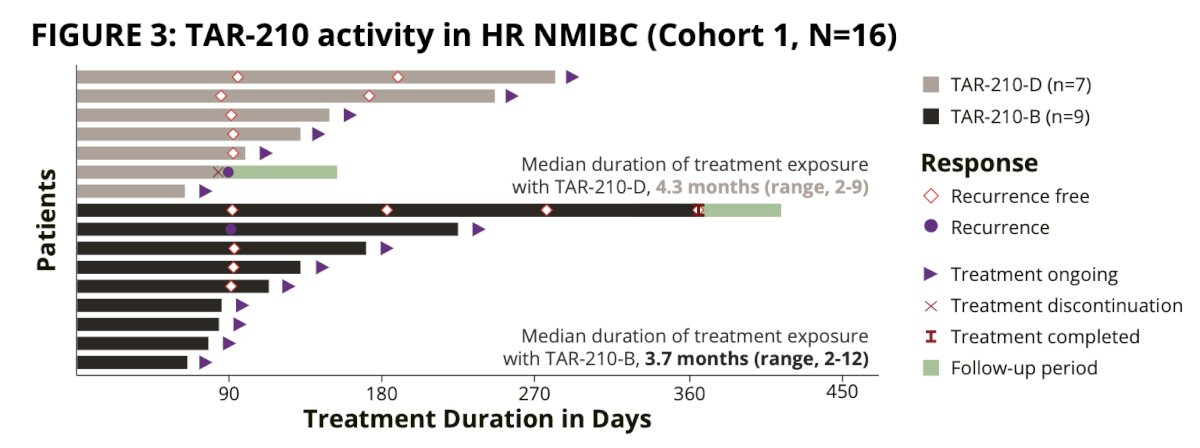
Median recurrence-free survival in Cohort 1 was non-estimable 95% CI 2.96 months to non estimable. In cohort 3, 15 patients had a response assessment and 13 achieved a complete response (rate: 87%):

All complete responses in cohort 3 are ongoing as of the clinical cutoff, with a median duration of response not reached. TAR-210 provided sustained erdafitinib release in urine over 90 days with very low plasma concentrations:

The most common treatment-related adverse events were grade 1/2 lower urinary tract adverse events. There were no dose-limiting toxicities. Two patients discontinued treatment due to adverse events of low-grade urinary symptoms, and one patient had serious adverse events of pyelonephritis and sepsis (unrelated to TAR-210). No deaths were reported:

Dr. Vilaseca concluded his presentation discussing the first safety and efficacy results of the TAR-210 erdafitinib intravesical delivery system in patients with non–muscle-invasive bladder cancer with select FGFR alterations with the following conclusions:
- TAR-210 shows promising clinical activity in patients with FGFR-altered high-risk and intermediate-risk non-muscle invasive bladder cancer with a recurrence-free rate of 82% in cohort 1 and a complete response rate of 87% in cohort 3
- TAR-210 was well tolerated with limited systemic toxicity
- These encouraging preliminary results support phase 3 studies of TAR-210 in FGFR-altered localized bladder cancer
Presented by: Felix Guerrero-Ramos, MD, PhD, FEBU, Department of Urology, Hospital Universitario 12 de Octubre, Madrid Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024


