(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a trials in progress poster session. Dr. Raj Satkunasivam presented an update of a phase II clinical trial of neoadjuvant sasanlimab plus stereotactic body radiation therapy (SBRT) as an in-situ vaccine for cisplatin ineligible muscle invasive bladder cancer (MIBC).
The use of neoadjuvant immune checkpoint inhibitor (ICI) therapy (e.g., PD1/L1 inhibitors) prior to radical cystectomy is an emerging paradigm in MIBC. Historically, pathologic complete responses (pCR) of approximately 40% have been observed with neoadjuvant cisplatin-based combination chemotherapy.1 Conversely, pCR of 25 – 40% have been more recently observed with neoadjuvant PD1/L1 inhibitor monotherapy.2,3 Sasanlimab (PF-06801591) is a humanized IgG monoclonal antibody that targets PD-1 selectively, for which there are both phase 1 data and ongoing phase 3 trials in early-stage urothelial carcinoma of the bladder. 4,5 In situ vaccination using radiation therapy may augment T-cell responses to tumor-specific antigens through immunogenic cell death. Recently, in non-small cell lung cancer, neoadjuvant SBRT + ICI demonstrated improved complete response rates.6
This trial aims to evaluate a novel strategy of combination neoadjuvant systemic ICI therapy plus SBRT directed to the primary tumor as an in-situ vaccine to improve locoregional control and decrease the risk of distant recurrence in cisplatin-ineligible MIBC patients.
The investigators hypothesized that combined ICI + SBRT treatment would be safe, and feasible and lead to improvement in the rate of pCR compared to currently available data on ICI monotherapy or platinum-based chemotherapy.
This is a phase II investigator-initiated, single-institution, prospective, open-label trial of neoadjuvant systemic sasanlimab in combination with SBRT to the tumor for cisplatin-ineligible
patients or those refusing cisplatin-based chemotherapy with MIBC prior to radical cystectomy. The study design is summarized below. Eligible patients will receive sasanlimab 300 mg subcutaneously on Day 1 of Cycle 1, followed by another injection of Day 1 of Cycle 2 concurrently with SBRT 24 Gy administered in 8 Gy fractions. Patients will be planned for a radical cystectomy 1–2 weeks later.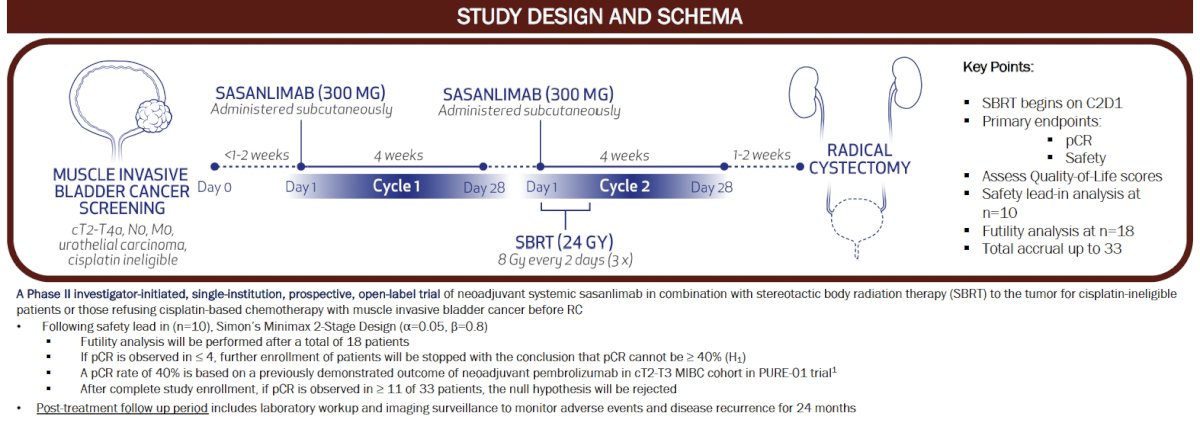
The key study eligibility criteria are summarized below. Of note, patients are required to have predominant (>50%) urothelial carcinoma histology. Patients with evidence of lymphadenopathy were study ineligible. Additional notable exclusion criteria include prior receipt of radiotherapy to the bladder, prior ICIs, and/or prior systemic therapy for bladder cancer.
The primary study objectives are as follows:
- The safety and feasibility of combined ICI/SBRT:
- Combination of sasanlimab and SBRT will be deemed both feasible and safe (composite outcome) if, after the treatment of 10 patients, ≥ 7 of 10 patients meet all the following feasibility criteria:
- Receive at least 1 of 2 planned doses of sasanlimab
- Receive at least 2 of 3 fractions SBRT
- Undergo radical cystectomy within 14 days of completing therapy (after end of cycle 2)
- And meet all the safety criteria, defined as not experiencing any following Common Terminology Criteria for Adverse Events (CTCAE) toxicities up to 4 weeks after the completion of RC:
- Hematologic toxicity ≥ Grade 4
- Non-hematologic toxicity ≥ Grade 3
- Non-hematologic toxicity ≥ Grade 2 lasting >1 week (except alopecia, emesis, and laboratory abnormalities)
- Combination of sasanlimab and SBRT will be deemed both feasible and safe (composite outcome) if, after the treatment of 10 patients, ≥ 7 of 10 patients meet all the following feasibility criteria:
- Pathologic complete response rate after neoadjuvant sasanlimab/SBRT, followed by RC
Additional study objectives/endpoints are as follows: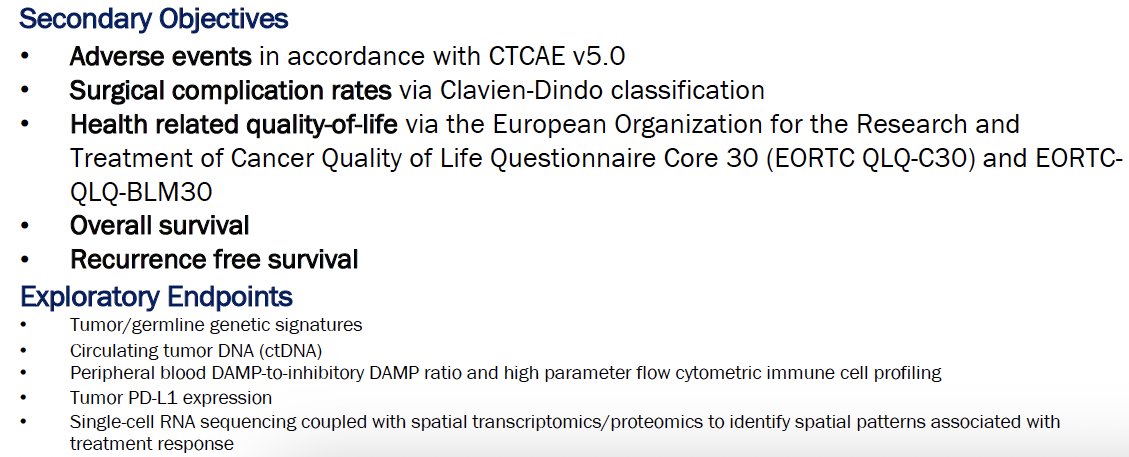
The anticipated total number of participants is 33. The trial opened for enrolment on February 15, 2022, and the first patient enrolled on March 30, 2022. The anticipated primary study completion date is January 2025.
Presented by: Raj Satkunasivam, MD, MS, Department of Urology, Houston Methodist Hospital, Houston, TX
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349(9): 859-866.
- Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol 2018 Dec 1;36(34):3353-3360.
- Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019 Nov;25(11): 1706-1714.
- Al-Khami AA, Youssef S, Abdiche Y, et al. Pharmacologic Properties and Preclinical Activity of Sasanlimab, A High-affinity Engineered Anti-Human PD-1 Antibody. Mol Cancer Ther. 2020;19(10): 2105-2116.
- Johnson ML, Braiteh F, Grilley-Olson JE, et al. Assessment of Subcutaneous vs Intravenous Administration of Anti-PD-1 Antibody PF-06801591 in Patients With Advanced Solid Tumors: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2019;5(7): 999-1007.
- Formenti SC, Rudqvist N, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12): 1845-1851.


