(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a non-muscle invasive bladder cancer (NMIBC) poster session of studies evaluating the benefits and harms of various treatment options. Dr. Charalampos Fragkoulis presented the final results of a phase II study evaluating intravesical durvalumab in patients with high-risk NMIBC who experienced Bacillus Calmette-Guerin (BCG) failure.
Intravesical BCG currently remains the standard-of-care, guideline recommended treatment of choice in the adjuvant setting for intermediate- and high-risk NMIBC due to its ability to reduce the risk of disease recurrence and, more importantly, disease progression.1-3 However, despite adequate BCG treatment, up to 50% of such patients will develop a BCG-refractory, relapsing, or failure state.4 Currently, radical cystectomy remains the gold standard approach in this setting.1 However, many patients are either unfit or refuse cystectomy. As such, bladder-sparing approaches in this setting are of utmost importance. To date, only three agents, valrubicin (Valstar®), pembrolizumab (Keytruda®), and nadofaragene firadenovec (Adstiladrin) have received FDA approval in the BCG unresponsive setting.
The aim of this study was to evaluate the efficacy and safety of intravesical durvalumab in patients with high-risk NMIBC who experience BCG failure, as well as evaluate in vivo data regarding durvalumab stability post-instillation.
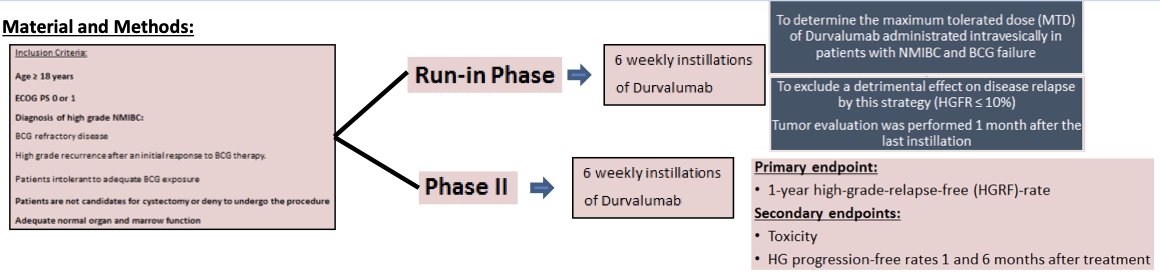
This trial included adult patients with an ECOG performance status of 0–1 who had a diagnosis of high-grade NMIBC meeting the following criteria:
- BCG-refractory disease
- High-grade recurrence after an initial response to BCG therapy
- Intolerance to BCG
Patients were either not candidates for or refused radical cystectomy. In the run-in phase, patients received 6 weekly instillations of durvalumab to determine the maximum tolerated dose (MTD) in this setting, as well as to exclude a detrimental effect on disease relapse with this strategy. In the phase II portion, the study endpoints were as follows:
- Primary: 1-year high-grade-relapse-free rate
- Secondary:
- Toxicity
- High grade progression-free rates 1 and 6 months after treatment
The median progression-free survival was 6 months.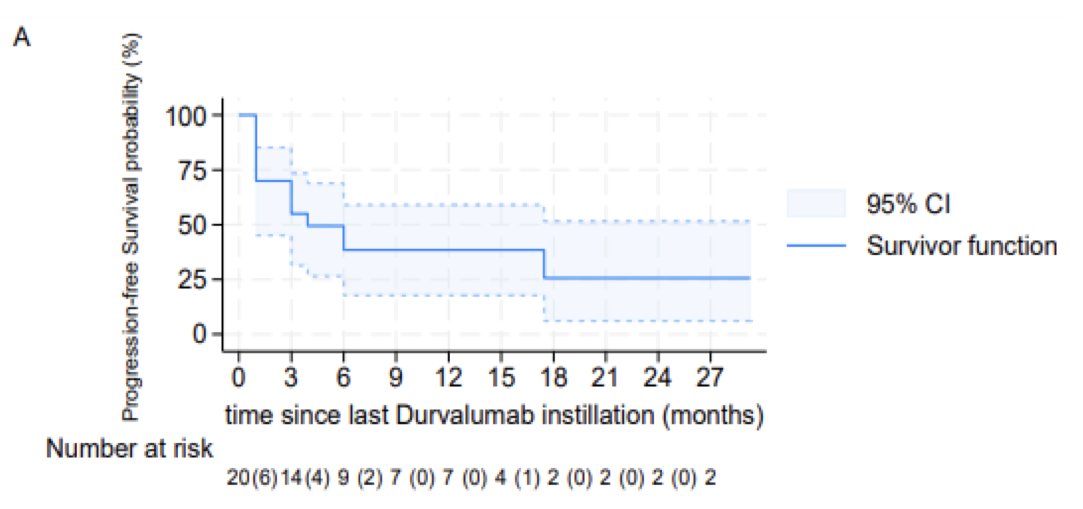
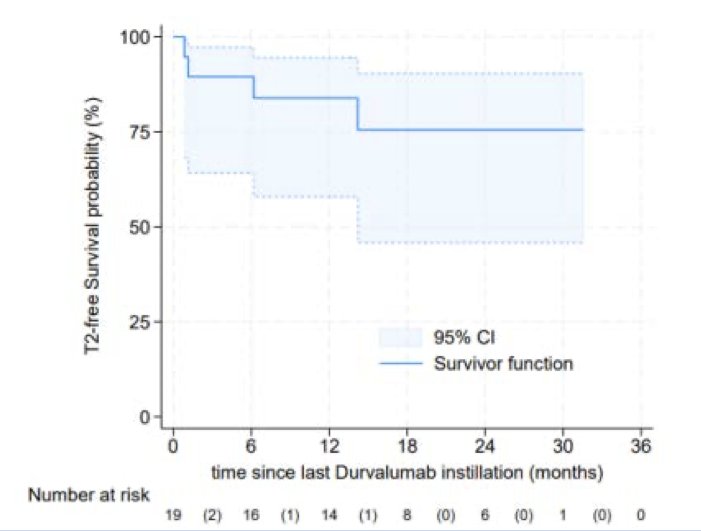
The median duration of freedom from muscle invasive disease progression was not reached. At two years, only 25% of patients had progressed to a muscle invasive stage.
The median cystectomy-free survival was approximately 20 months.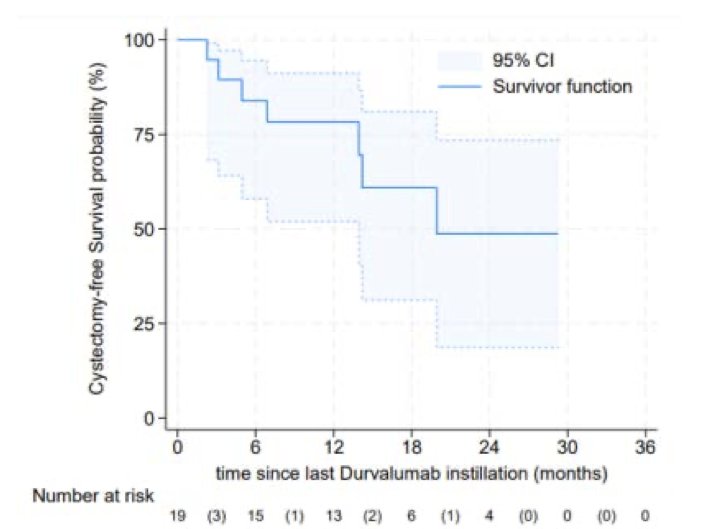
One patient experienced a grade 5 COVID-19 infection during treatment, with all other adverse events classified as grade 1 in severity.
Based on these results, the investigators concluded that intravesical durvalumab administration at 1,000 mg is feasible in patients with BCG-failing high-risk NMIBC with negligible toxicity and activity consistent with intravenous immune checkpoint inhibitors or intravesical nadofaragene firadenovec gene therapy in the same setting.
Presented by: Charalampos Fragkoulis, MD, MSc, Department of Urology, General Hospital of Athens, Athens, Greece
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Review. 2020;1(1):CD011935.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 Update. Eur Urol. 2019;76(5):639–57.


