(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a session on navigating urothelial carcinoma from innovative diagnostics to therapeutic strategies, and a presentation by Dr. Husney Mahmud discussing a first in human trial of non-thermal atmospheric plasma ablation for intermediate risk non-muscle invasive bladder cancer.
Bladder cancer is one of the most common malignancies, with most patients having low-grade and low-stage tumors that do not progress to metastasis. However, these tumors frequently recur after local resection and intra-vesical therapy, requiring repeated transurethral resection with the need for hospitalization. There is a need for minimally invasive outpatient alternatives for tumor resection for low-volume recurrence. Non-thermal atmospheric plasma is a novel procedure that can deliver ablative energy transurethral to the bladder and target the lesions directly without the need for anesthesia and hospitalization.
Patients in this trial had recurrent low to intermediate-risk non-muscle invasive bladder cancer who had up to 7 tumors at a maximal tumor size of 2 cm. Transurethral ablation with non-thermal atmospheric plasma was performed under general anesthesia, using helium insufflation of the bladder at a pressure of up to 12 mmHg. The investigators applied non-thermal atmospheric plasma to each tumor for 3-6 minutes according to a dose escalation protocol: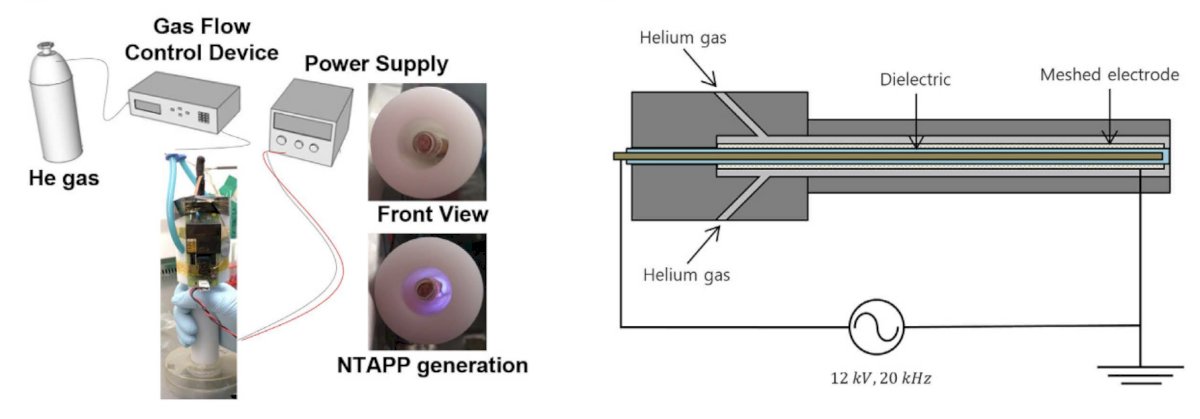
The clinical and cystoscopic data of the tumor location before, during, and after treatment were recorded with a follow-up of up to one year. Study endpoints included the incidence, severity, and causality of major adverse tissue effects and the maximum tolerated dose of non-thermal atmospheric plasma for bladder tumors. The exploratory endpoint was treatment efficacy, defined by the absence of visible tumor at the previous non-thermal atmospheric plasma-treated site 3 months after treatment.
There were 27 patients with 42 lesions treated with the non-thermal atmospheric plasma system and had at least one follow-up visit, with a median follow-up of 3 months (range 3 weeks to 1 year). There was no evidence of non-thermal atmospheric plasma-related toxicity noted among the different doses. In a few of the cases, lesions were resected transurethrally due to the inaccessibility of the tumor by the non-thermal atmospheric plasma probe. All patients were discharged after catheter removal according to the protocol, most of them on the same day of the procedure. The only procedure-related complication was extraperitoneal perforation after TURBT for a lesion in the anterior wall that was not reached by the non-thermal atmospheric plasma probe. The initial 3-month treatment complete response rate was 85%. In 5 of the patients, an additional non-thermal atmospheric plasma treatment was performed for new lesions detected during the follow-up visits. Taken together, 59 lesions were treated by non-thermal atmospheric plasma throughout the study, of which 50 had a complete response (85%). During the follow-up period, transurethral resection of bladder tumor was performed for non or partial responsive patients (n = 4, 15%) or newly detected tumor (n = 3, 11%).
Dr. Mahmud concluded his presentation discussing a first in human trial of non-thermal atmospheric plasma ablation for intermediate risk non-muscle invasive bladder cancer with the following statements:
- Non-thermal atmospheric plasma ablation has a high safety profile and a significant potential as an ambulatory alternative to TURBT for low-volume, low-to-intermediate risk non-muscle invasive bladder cancer
- A pivotal trial will evaluate the efficacy of this therapy in this patient population
Presented by: Husney Mahmud, Sheba Tel-HaShomer Medical Centre, Ramat Gan, Israel
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024


