(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a plenary session on living with advanced kidney cancer and urothelial cancer, and a state of the art lecture by Dr. Andrea Necchi discussing whether there is a new standard treatment of metastatic bladder cancer based on EV-302/KEYNOTE-A39. Dr. Necchi started his presentation by highlighting the first-line therapy for metastatic urothelial carcinoma prior to the ESMO 2023 meeting, emphasizing both negative and positive trials in this disease space:

Dr. Necchi notes that avelumab has solidified the position of maintenance therapy after not progressing on first line chemotherapy. Presented at ESMO 2023, and subsequently published in the Journal of Clinical Oncology, Powles et al.1 reported results of the JAVELIN Bladder 100 trial after ≥2 years of follow-up. At the time of the data cutoff for this analysis (June 4, 2021), the median follow-up was 38 months for patients receiving avelumab versus 39.6 months for those in the control arm. Of note, almost 20% of patients had received ≥2 years of avelumab maintenance therapy. In this extended analysis, OS remained prolonged with avelumab, with a median survival of 23.8 months (95% CI 19.9 – 28.8) versus 15 months (95% CI 13.5 – 18.2) for the control arm (HR 0.76, 95% CI 0.63 – 0.91):

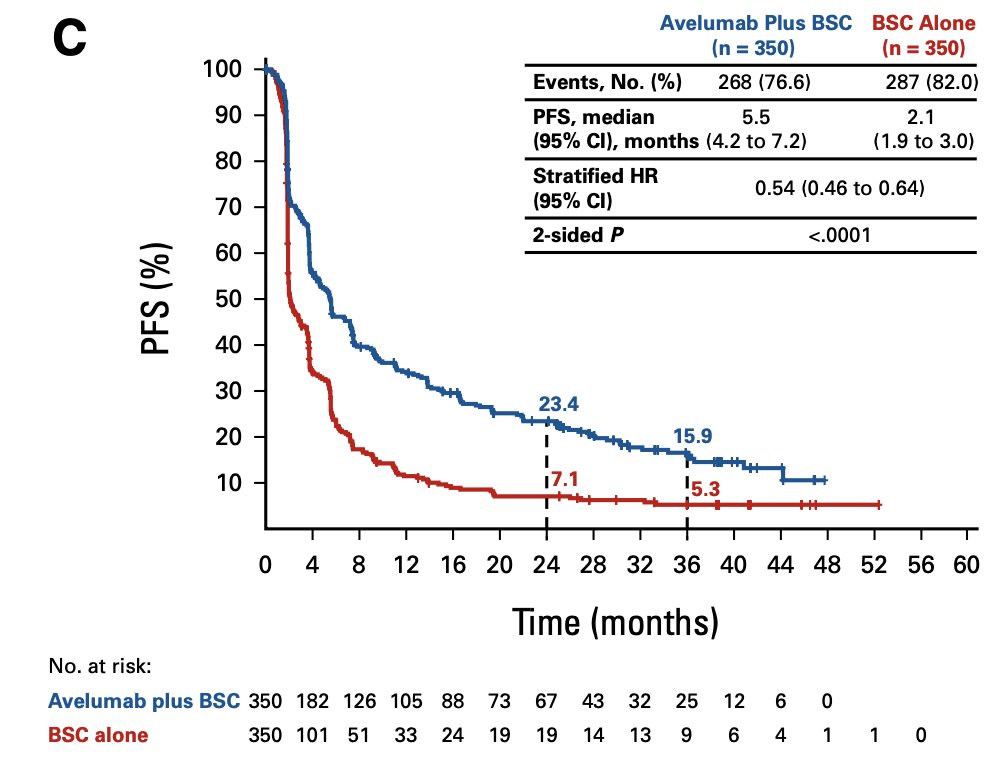
Additionally, investigator-assessed progression free survival remained prolonged for patients receiving avelumab, with a median of 5.5 months (95% CI 4.2 – 7.2) versus 2.1 months (95% CI 1.9 – 3.0) in the control arm (HR 0.54, 95% CI 0.46 – 0.64):
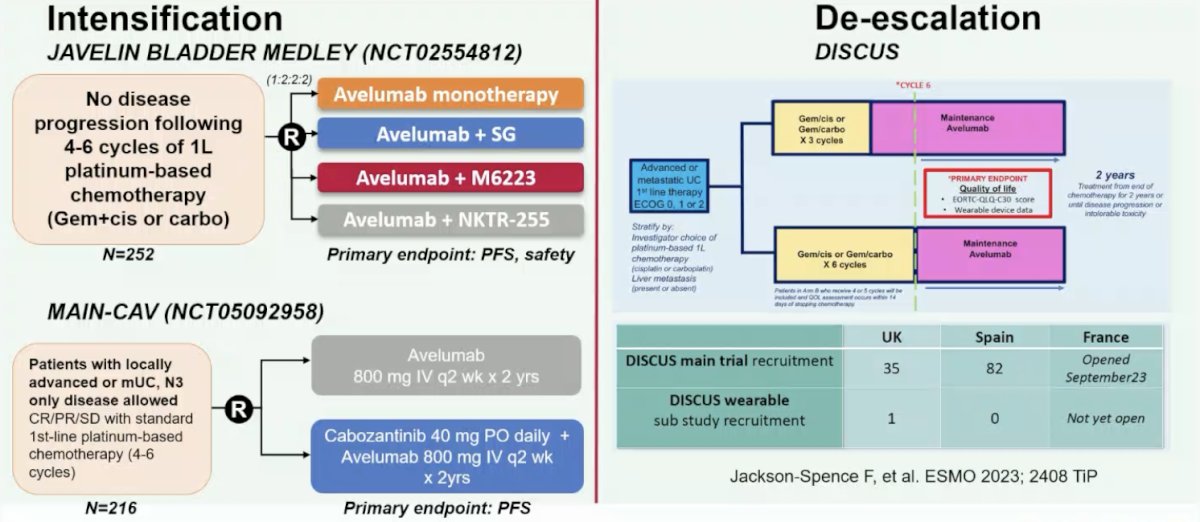
Dr. Necchi notes that there are two ways of pursuing clinical research in the maintenance setting after first-line chemotherapy:
- Intensification: being assessed in the JAVELIN BLADDER MEDLEY trial and the MAIN-CAV trial
- De-intensification: being assessed in the DISCUS trial
Dr. Necchi then discussed the CheckMate-901 trial, of which 608 cisplatin-eligible patients underwent 1:1 randomization to either:
- Nivolumab 360 mg on D1 + gemcitabine (1,000 mg/m2) on D1/8 + cisplatin (70 mg/m2) on D1 in 3-week cycles, up to a total of 6 cycles
- Nivolumab maintenance at 480 mg every 4 weeks was continued as maintenance therapy in responders until progression, unacceptable toxicity, withdrawal, or up to 24 months
- Gemcitabine + cisplatin at same doses/schedule/cycles
The trial design for CheckMate 901 is as follows:

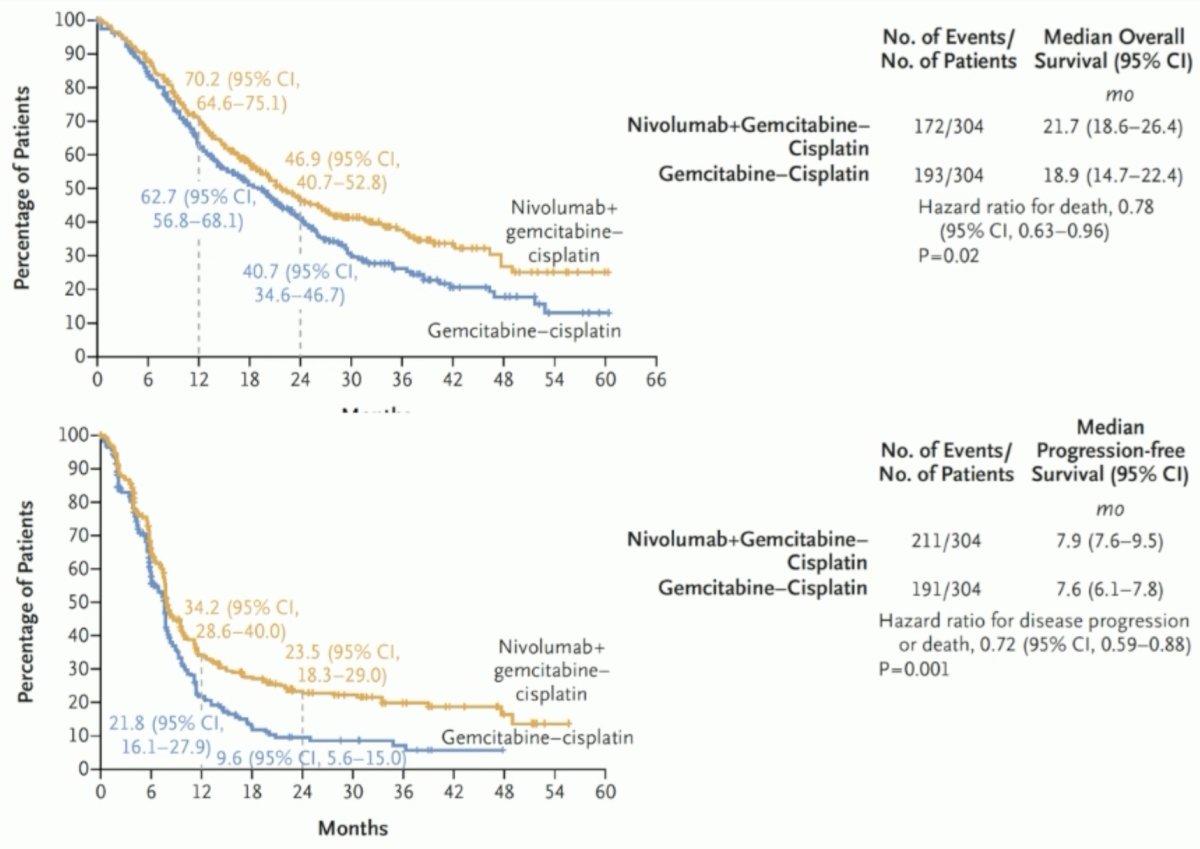
The median patient age was 65 years and 25% of patients had upper tract disease. Overall, 37% of patients had a high tumor PD-L1 expression, defined as ≥1%, and 21% of patients had liver metastases. The median study follow-up was 33.6 months, and the study met its primary endpoint of overall survival, with a median improvement of nearly 3 months (21.7 versus 18.9 months; HR: 0.78, 95% CI: 0.63 – 0.96, p=0.017). The other co-primary endpoint of PFS per BICR was also significant with improvement in median PFS from 7.6 to 7.9 months (HR: 0.72, 95% CI: 0.59 – 0.88, p=0.0012):
Other impressive outcomes favoring nivolumab + gemcitabine + cisplatin are the 21.7% complete response rate and the median duration of 37.1 months (95% CI 18.1 – NE) versus 13.2 (95% CI 7.3-18.4) for gemcitabine + cisplatin. The ongoing phase 3 NILE trial is assessing immunotherapy + chemotherapy in first line metastatic urothelial carcinoma, with a planned sample size of 1,292 patients:

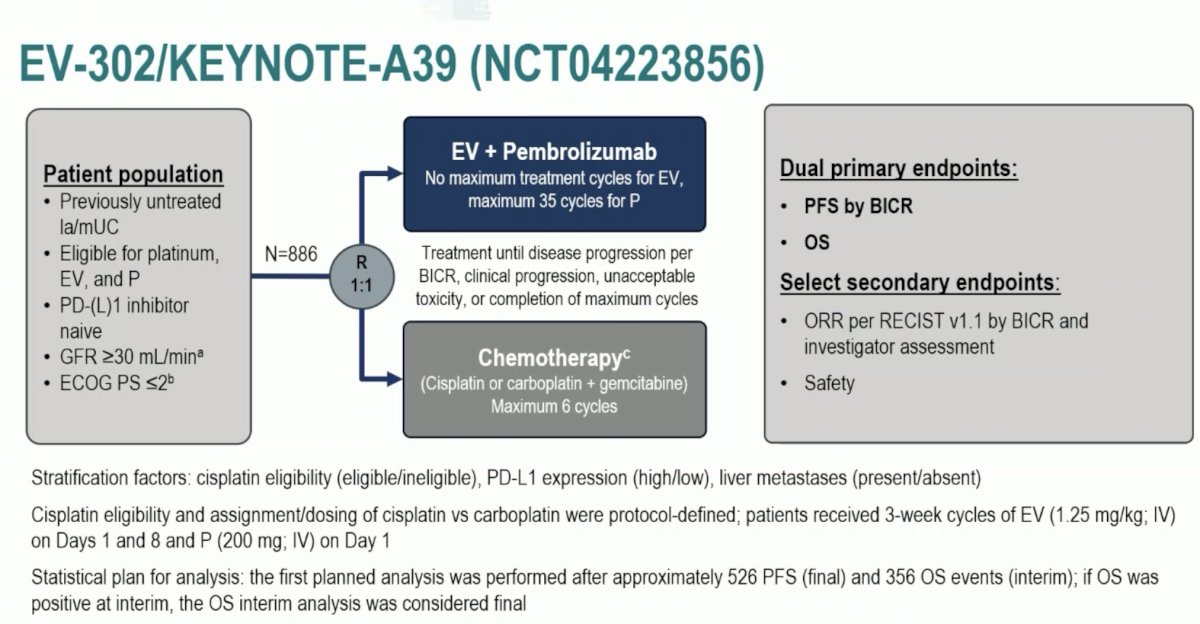
Next, Dr. Necchi discussed the landmark trial presented at ESMO 2023 and subsequently published in The New England Journal of Medicine, the EV-302/KEYNOTE-A39 trial [3]. Patients in this trial were randomized 1:1 to either enfortumab vedotin + pembrolizumab (n=442) or gemcitabine + cisplatin/carboplatin (n=444) for a maximum of 6 cycles. The dual primary endpoints were progression-free survival, assessed via blinded independent central review (BICR), and OS. The study design is as follows:
Compared to platinum-based chemotherapy, enfortumab vedotin + pembrolizumab prolonged progression-free survival from a median of 6.3 months to 12.5 months (HR: 0.45, 95% CI: 0.38 – 0.45, p<0.001):

OS was nearly doubled in the enfortumab vedotin + pembrolizumab arm, with median survivals of 31.5 and 16.1 months, respectively (HR: 0.47, 95% CI: 0.38 – 0.58, p<0.00001). These survival benefits were observed despite a higher proportion of patients in the chemotherapy arm receiving a subsequent systemic therapy (66.2% versus 28.9%):

Treatment-related adverse events (TRAEs) were consistent with the known safety profiles of each of the drugs. Serious TRAEs occurred in 27.7% of patients in the enfortumab vedotin + pembrolizumab arm, compared to 19.6% with chemotherapy. TRAE leading to death occurred in 4 patients (0.9%) in each arm. Overall, grade 3+ adverse events occurred in 56% and 70% of patients in the enfortumab vedotin + pembrolizumab and chemotherapy arms, respectively. The most common enfortumab vedotin TRAEs were skin reactions, peripheral neuropathy, ocular disorders, and hyperglycemia:

Last month, the ESMO Clinical Practice Guideline published an interim update on first-line therapy in advanced urothelial carcinoma [4], highlighting a treatment algorithm stratified by the availability of enfortumab vedotin + pembrolizumab:

Dr. Necchi then listed the pros and cons of choosing enfortumab vedotin + pembrolizumab and the pros and cons of choosing nivolumab + gemcitabine + cisplatin:
- Enfortumab vedotin + pembrolizumab:
- Almost doubled the median overall survival versus chemotherapy (OS 31.5 months)
- Benefit obtained regardless of baseline patient and disease characteristics
- Total treatment costs may not be easily affordable by healthcare systems
- Criteria for enfortumab vedotin eligibility are not well defined
- Some enfortumab vedotin-related adverse events are unpredictable making enfortumab vedotin + pembrolizumab performance transition to community oncology not immediate
- No biomarker of enfortumab vedotin + pembrolizumab benefit
- Possibly influenced by earlier use of pembrolizumab or enfortumab vedotin + pembrolizumab in the perioperative setting
- Second-line therapy is very much complicated
- Nivolumab + gemcitabine + cisplatin:
- Overall survival benefit with a median overall survival of 21.7 months
- Overall treatment burden is de-escalated versus enfortumab vedotin + pembrolizumab (maintenance nivolumab versus enfortumab vedotin + pembrolizumab)
- The complete response rate is compelling
- Median duration of complete response is compelling
- Characterizing the outlier complete response patients is of paramount importance
- Possibly influenced by earlier use of nivolumab or nivolumab + gemcitabine + cisplatin in the perioperative setting
- Possibility of sequencing an antibody drug conjugate in the second-line
There are several ongoing cisplatin-eligible and cisplatin-ineligible trials:
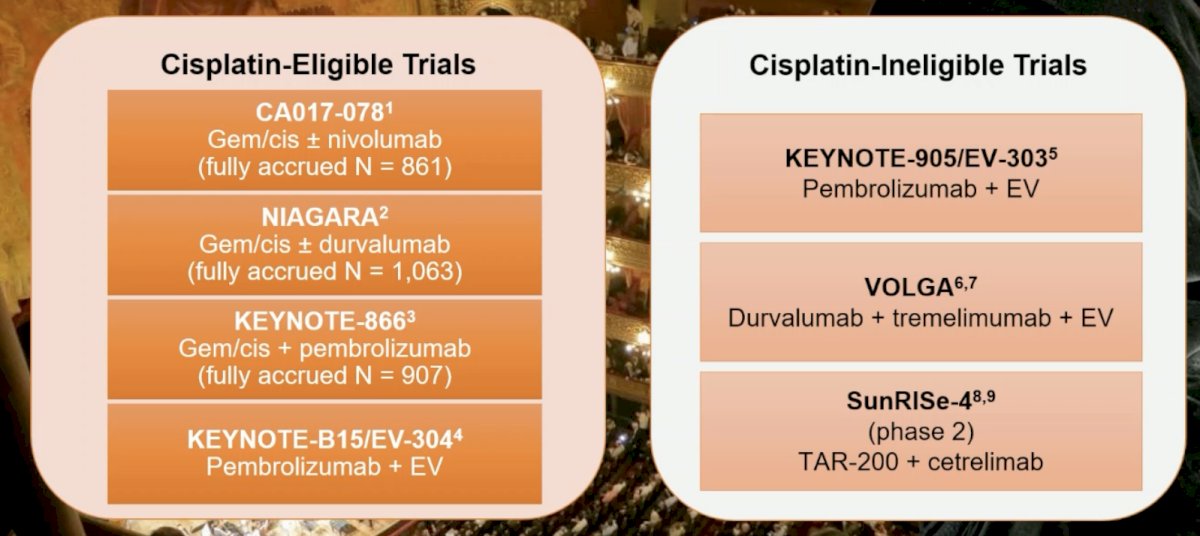
Importantly, trastuzumab deruxtecan received accelerated approval for HER2 3+ solid tumors on April 5, 2024, based on results from the objective response rates across tumor cohorts in the DESTINY-PanTumor02 trial. This may have implications for bladder cancer patients who had 39% IHC 3+ in this trial. There are several other ADCs under investigation in metastatic urothelial carcinoma. Two trials are targeting HER2, including disitamab vedotin + toripalimab and trastuzumab deruxtecan in the DESTINY-PanTumor02 trial. One trial targeting TROP2 is assessing sacituzumab govitecan, which received FDA approval for refractory metastatic urothelial carcinoma based on the phase 2 TROPHY-U-01 trial [4]. The phase 3 TROPiCS-04 trial is underway.
Dr. Necchi concluded his presentation by discussing whether there is a new standard treatment of metastatic bladder cancer by highlighting the typical patient to treat in the first-line metastatic setting in 2024 and beyond:

Presented by: Andrea Necchi, MD, IRCCS Ospedale San Raffaele, Milan, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- Powles T, Park SH, Caserta C, et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. J Clin Oncol. 2023;41: 3486-3492.
- Van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023 Nov 9;389(19):1778-1789.
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10)875-888.
- Tagawa ST, Balar AV, Petrylak DP, et al. Metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021 Aug 1;39(22):2474-2485.


