(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Professor Peter Mulders discussed why molecular imaging evaluation is the ‘way to go’ for the initial staging of renal cell cancer (RCC).
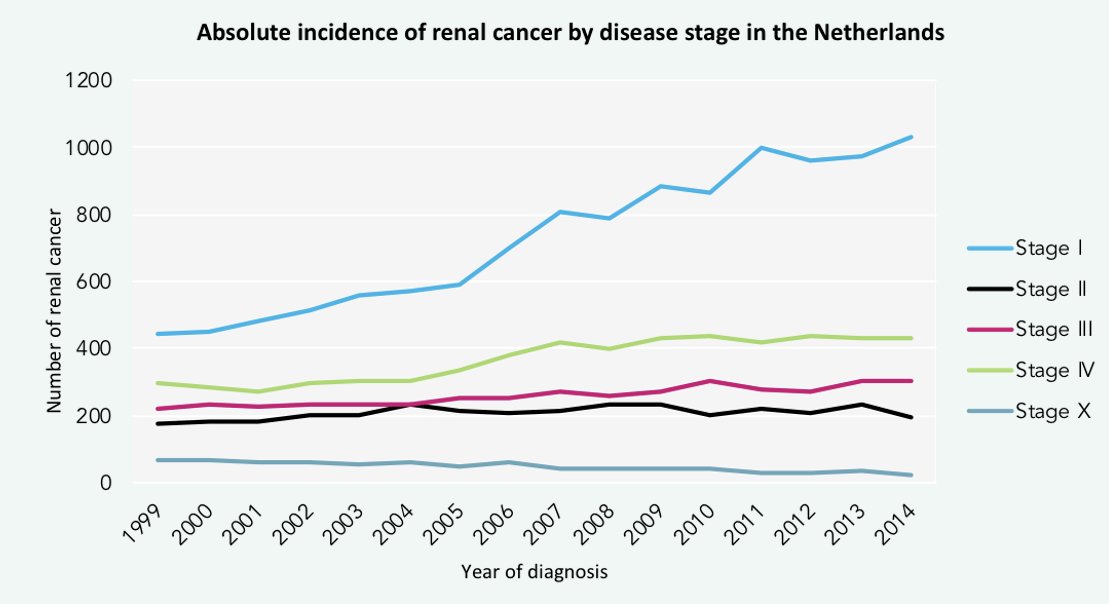
There has been a stage migration for RCCs in the Netherlands. Between 1999 and 2014, there has been a steady rise in the incidence of stage 1 renal malignancies, likely owing to the increased utilization of cross-sectional imaging in clinical practice. As such, small renal masses are emerging as a clinical entity of increased clinical significance.
There are important pathologic differences between renal tumor subtypes that are reflected in distinct morphologic features and underlying genetic mutations. These differences could be leveraged to develop non-invasive imaging tools to help distinguish between the different histologic subtypes.
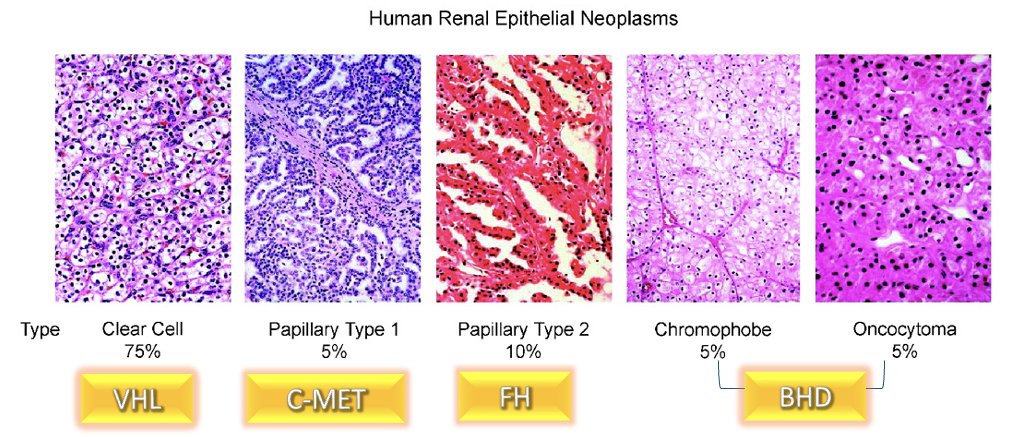
There has long been an unmet clinical need for a non-invasive approach to the diagnosis and characterization of clear cell RCCs, which account for 75% of all human renal epithelial neoplasms and cause 90% of deaths1 Anatomic imaging cannot reliably distinguish between benign and malignant renal masses. Renal mass biopsy remains invasive, is performed infrequently, and is often non-diagnostic. Furthermore, among patients with small renal masses on active surveillance, clear cell RCC subtype is associated with the most rapid linear growth rate.2 Accordingly, it becomes clear that identification of a clear cell RCC-specific cell surface marker allowing for targeted imaging techniques in this disease setting is of utmost importance.
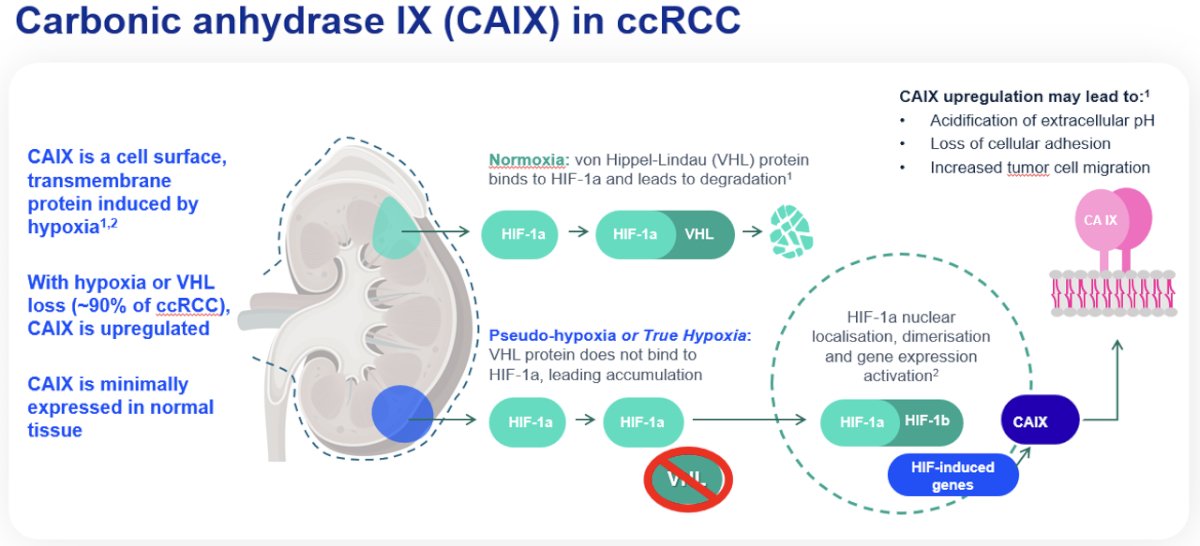
Carbonic anhydrase IX (CAIX) is a transmembrane glycoprotein involved in oxygen sensing pathways and has a functional role in tumor acid/base regulation. It is expressed in many hypoxic solid tumors and under hypoxic conditions activates HIF-1 mediated signaling cascades, leading to the activation of hypoxia-regulated genes. This leads to cell metabolism reprogramming (‘glycolic switch’), with the production and export of lactate, which leads to a decline of the extracellular environment pH. The acidic extracellular space promotes tumor cell invasiveness, with hypoxic conditions correlated with disease progression and therapy resistance. CAIX has low expression levels in normal tissues, making it an attractive potential ‘pan-cancer’ target. Significantly, CAIX is expressed in 90% of clear cell RCC tumors.
89Zr-DFO-girentuximab (TLX250-CDx) is an antibody-conjugate PET imaging tracer that specifically targets CAIX. Girentuximab is an IgG1 kappa light chain chimeric monoclonal antibody that specifically binds the CAIX and is subsequently internalized. Girentuximab has been demonstrated to be safe in numerous studies. Significantly, it is excreted via the hepato-biliary route which allows for the optimization of renal visualization. 89Zr is the ‘payload’ positron emitter component. It is also hepatically cleared with a half-life of 3.3 days. This conjugate (89Zr + girentuximab) has previously demonstrated feasibility for the imaging of CAIX positive tumors, using both SPECT and PET imaging modalities. It has a favorable safety profile, and given its clearance characteristics, allows for imaging scheduling flexibility (4 to 7 days after administration).

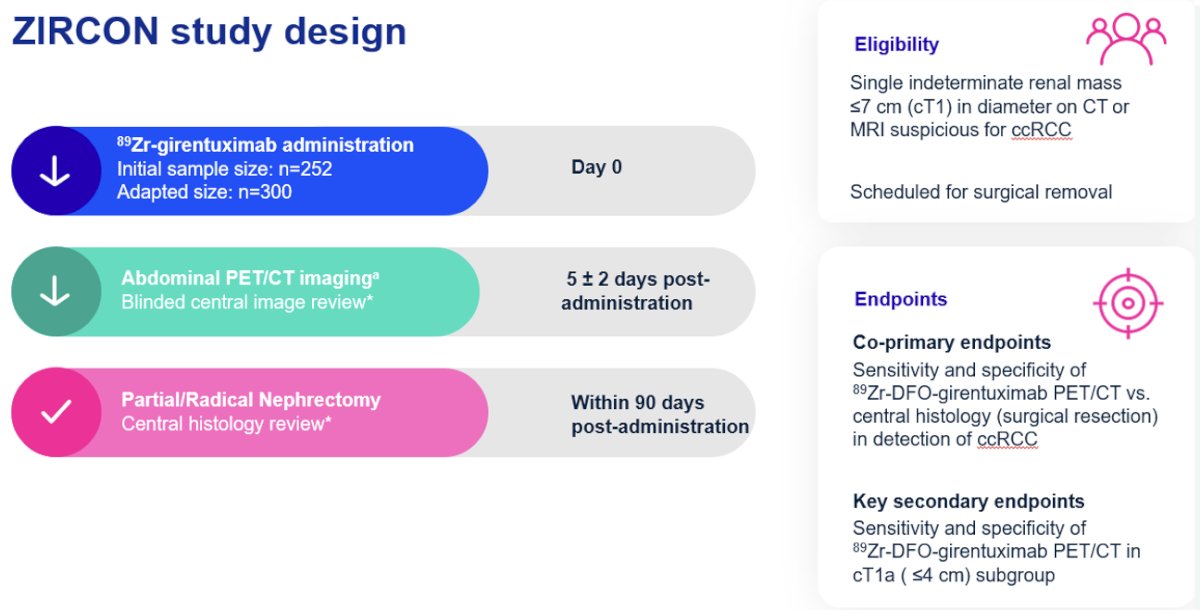
ZIRCON is an international, multicenter trial conducted at 36 sites across nine countries between 2019 and 2022. This trial included patients with a single, indeterminate cT1 renal mass (i.e., ≤7 cm) suspicious for clear cell RCC, detected on CT or MRI. All patients were scheduled for surgical removal (histologic reference standard). Patients received 89Zr-girentuximab on day 0. They subsequently underwent abdominal PET/CT imaging five days (+/- 2) following tracer administration. PET/CT imaging findings were evaluated using a blinded central imaging review. All patients were subsequently planned for partial or radical nephrectomy, with a central histology review of surgical specimens, within 90 days of tracer administration.
The co-primary endpoints were the sensitivity and specificity of 89Zr-DFO-girentuximab PET/CT for the detection of clear cell RCC, using the surgically resected mass as the reference standard. The key secondary endpoints were sensitivity and specificity in the cT1a (≤4cm) subgroup. All scans were assessed by three independent readers. The study would be deemed ‘positive’ if the lower boundaries of the 95% confidence intervals for both sensitivity and specificity exceeded 70% for two of the three independent readers.
Three hundred patients received the tracer (safety analysis set), of whom 284 were included in the primary analytic set (179 cT1a). Of the 288 evaluable patients (4 subsequently excluded), 67% had clear cell RCC. Other histologic subtypes included papillary RCC (15%), chromophobe RCC (8%), and oncocytomas (3%).
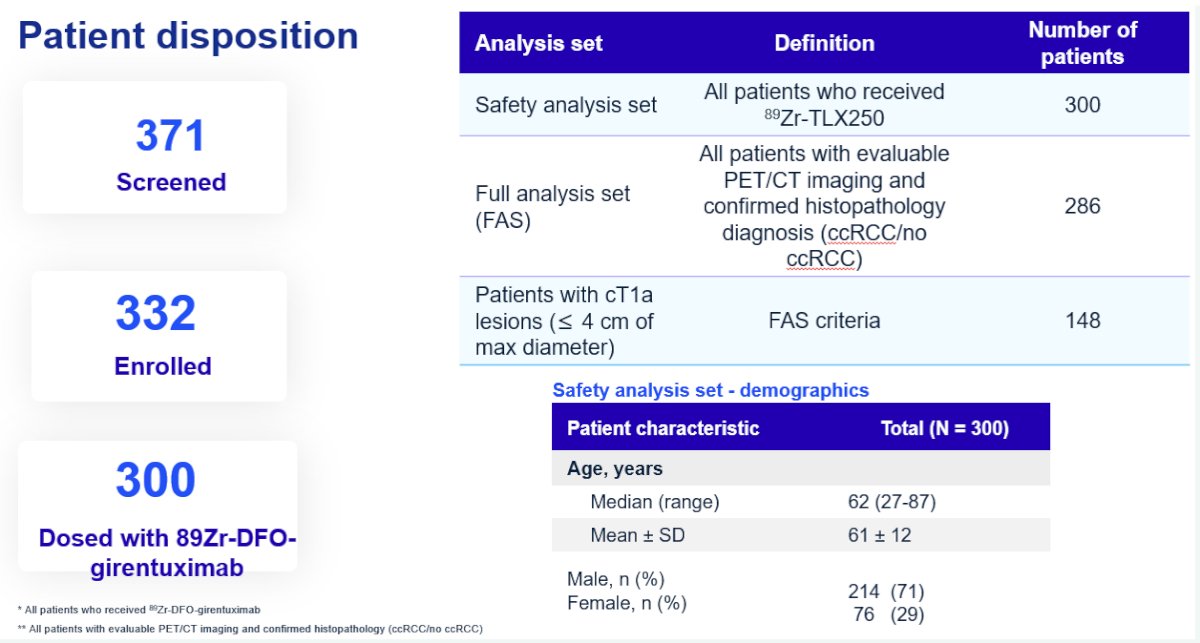
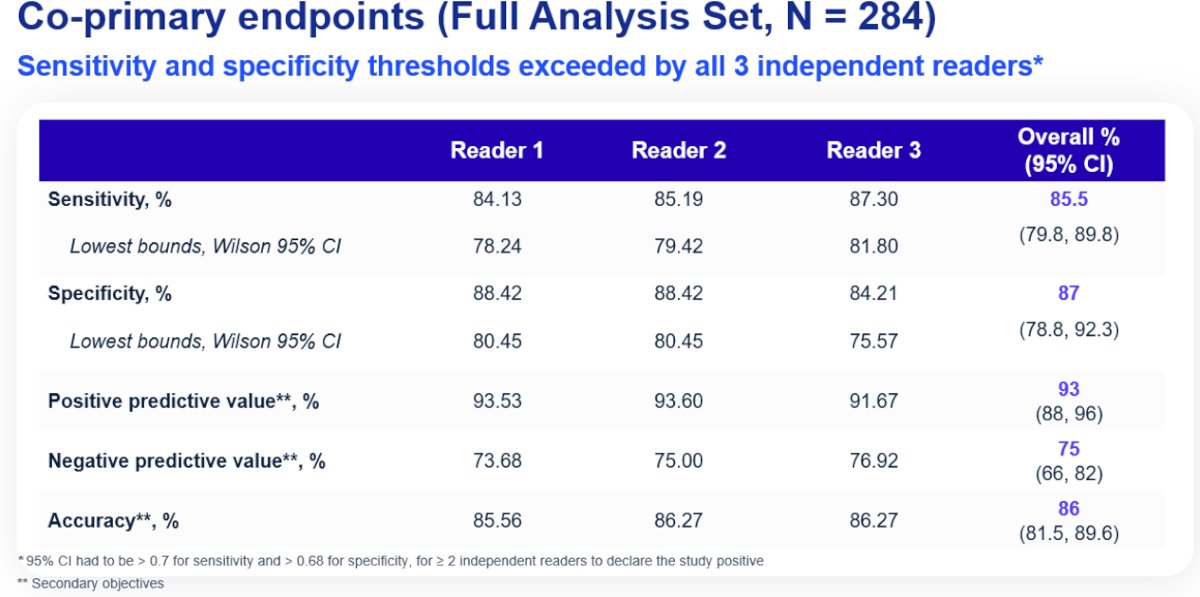
For the co-primary endpoints of sensitivity and specificity in the full analysis set, the corresponding values were 85.5% and 87%, respectively. The positive and negative predictive values were 93% and 75.2%, respectively. The overall accuracy in cT1 masses was 86%. As demonstrated below, we note that the lower boundary of all 95% confidence intervals for each reader exceeded 70%. Thus, this study met its co-primary endpoints.
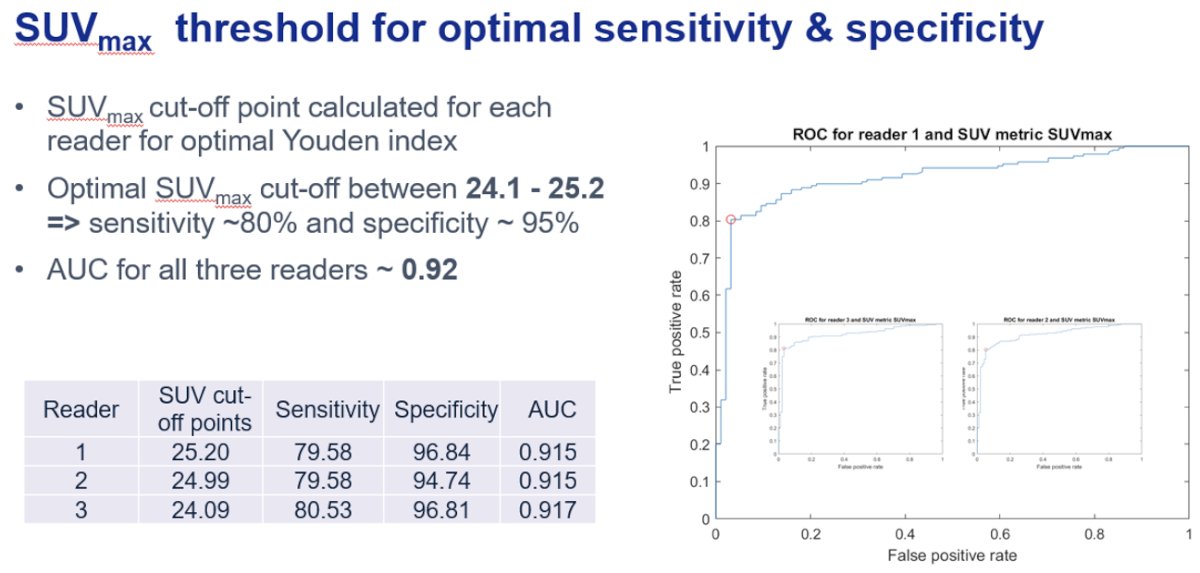
Patients with PET-positive clear cell RCC had the highest average standardized uptake values (SUV) and tumor-to-background ratios (TBR). The SUVmax, SUVpeak, TBR max, and TBR peak values for true positive lesions were 56, 40.2, 12.1, and 8.6, respectively.
The SUVmax cut-off point was calculated for each reader for optimal Youden index. The optimal SUVmax cut-off was determined to be between 24.1 and 25.2, corresponding to a sensitivity of ~80% and specificity of ~95%.
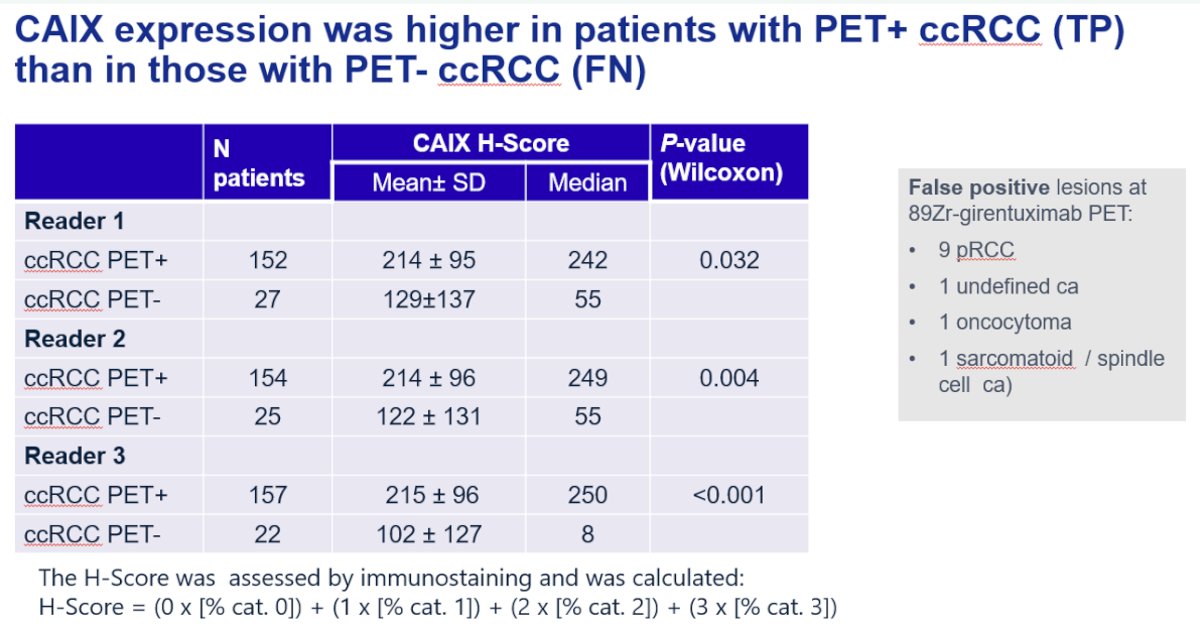
CAIX expression was higher in patients with PET+ clear cell RCC (i.e., true positives) compared to those with PET- clear cell RCC (i.e., false negatives).
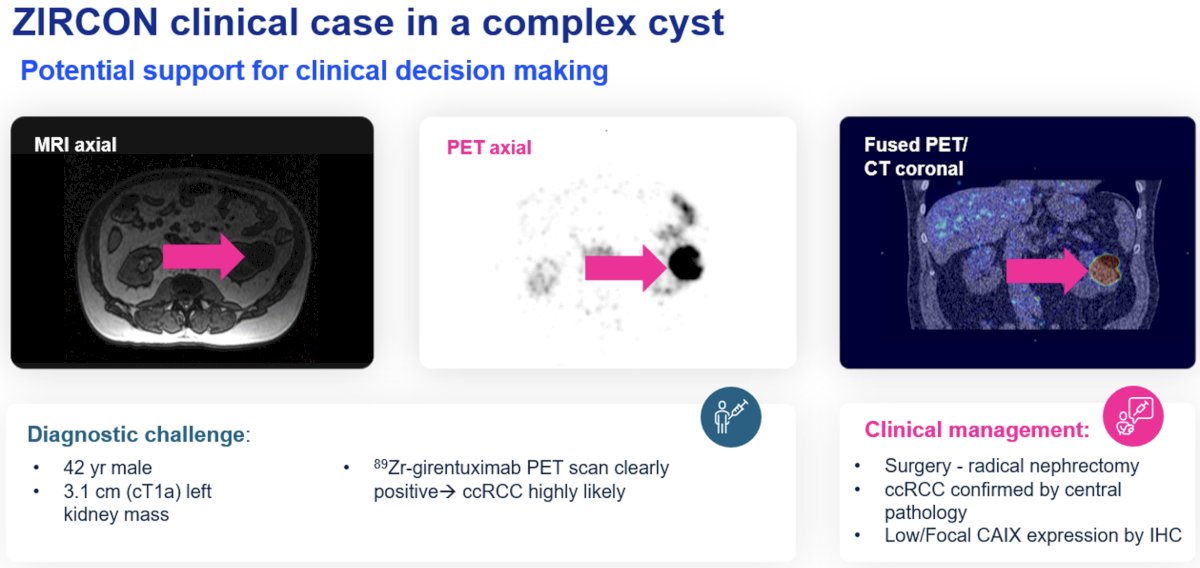
Professor Mulders next presented a clinical case for which ZIRCON was particularly helpful. This was a case of a 42-year-old male with a 3.1 cm left renal complex cyst. Renal mass biopsies are particularly challenging for such complex renal cysts given their low diagnostic yield in this setting (targeting the solid component is challenging and may not be representative). The 89Zr-girentuximab PET scan was positive, indicating the presence of clear cell RCC. The patient underwent a subsequent radical nephrectomy with clear cell RCC pathology confirmed on final pathology.
He next presented another case of a 57-year-old-male with a 1 cm lesion incidentally found on cross sectional imaging. This patient was being considered for active surveillance versus definitive management. The patient underwent a 89Zr-girentuximab PET that was clearly positive, indicating that clear cell RCC was highly likely. The patients subsequently underwent a partial nephrectomy confirming the histologic diagnosis. While active surveillance is a reasonable option in this setting, this case highlights how the results of this scan can help guide treatment decision making in this young patient who strongly preferred definitive management in the setting of likely malignancy.

Professor Mulders concluded with the following with regard to the ZIRCON trial results and its impact on molecular imaging of clear cell RCC:
- These positive results suggest that 89Zr-DFO-girentuximab improves the identification of primary clear cell RCC, compared to cross-sectional imaging
- 89Zr-DFO-girentuximab has the potential to improve management by aiding risk stratification, selecting appropriate patients for treatment, or suggesting where further imaging/biopsy could be indicated
- 89Zr-DFO-girentuximab holds promise to improve staging in clear cell RCC, act as a therapeutic target for radiopharmaceuticals, and potentially image other solid tumors (true hypoxia) – all of which are ongoing initiatives
Presented by: Professor Peter Mulders, MD, PhD, Chairman of the Department of Urology at the Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024References:
- Linehan WM, Pinto PA, Srinivasan R, et al. Identification of the genes for kidney cancer: opportunity for disease-specific targeted therapeutics. Clin Cancer Res. 2007;13(2 Pt 2): 671s-691s.
- Finelli A, Cheung DC, Al-Matar A, et al. Small Renal Mass Surveillance: Histology-specific Growth Rates in a Biopsy-characterized Cohort. Eur Urol, 2020;78: 460-467.


