(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Professor Jean-Christophe Bernhard discussed why evaluation with conventional imaging is ‘enough’ for the initial staging of renal cell carcinoma (RCC).
Conventional imaging for renal cell carcinoma includes ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) scans. These tools allow for the detection, tumor characterization, and staging of renal masses. Based on a 2019 systematic review, it appears that CT has 88% sensitivity and 75% specificity for the detection of renal masses, whereas MRI has corresponding values of 88% and 89%, respectively.1
What about the characterization of cystic masses? Based on the current evidence, it appears that MRI outperforms CT in this setting with a sensitivity of 71% and specificity of 91%. An emerging tool for the enhanced characterization of these lesions is contrast-enhanced ultrasound, which allows for the improved detection of lesion septa, evaluation of septa/wall thickness, and characterization of fine enhancement of small structures. This tool has a reported sensitivity of up to 100% and specificity of 97%, with a negative predictive value of 100% for detecting the solid component in cystic lesions.
Overall, the major limitations to conventional imaging include:
- Poor predictive accuracy (32 to 64%) for presence of pT3a disease
- Positive predictive value of <45% for detection of pN+ disease
Can these shortcomings be overcome with novel imaging tools? Machine-learning approaches are being increasingly utilized to predict the presence of pT3a disease. Dr. Bernhard’s group recently published the results of an analysis utilizing the French multi-institutional kidney cancer database UroCCR, including 4,395 patients who underwent either a partial or radical nephrectomy between 2000 and 2019. Seven machine-learning algorithms were applied to the cohort after a training/testing split to develop a predictive model for upstaging to pT3a. Survival curves for disease-free survival and overall survival rates were compared between partial and radical nephrectomy after G-computation for pT3a tumours. Among these 4,395 patients, 667 patients (15%) were upstaged to pT3a disease. The UroCCR-15 predictive model demonstrated an area under the receiver-operating characteristic curve of 0.77.2
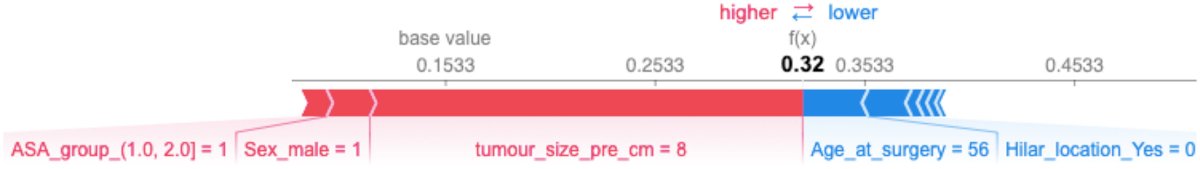
Another emerging tool is radiomics, which involves computational methods to extract quantitative metrics, such as shape, size, and texture from any standard clinical image dataset, such as CT or MRI. Currently, CT has a sensitivity and accuracy of 86% and 74%, respectively, for differentiating clear cell RCC from oncoytoma,3 with an accuracy of 87% for differentiating clear cell from non-clear cell RCC subtypes.4 Radiomics has been proposed as a technique to overcome these limitations.
In 2023, Garnier et al. published the results of a CT-based clinical, radiological, and radiomic machine learning model to help differentiate malignant from non-malignant renal masses. A total of 122 patients with 132 renal lesions, including 111 RCCs and 21 benign tumors were evaluated. Factors significantly associated with malignancy included: unilaterality, necrosis, lower values of tumor/cortex ratio between arterial and portal times, and higher variation of tumor/cortex ratio between arterial and portal times. A total of 35 radiomics features were selected, and "intensity mean value" was associated with RCCs in multivariate analysis (OR = 0.99). Following, ten-fold cross-validation, a C5.0Tree model was retained for its predictive performances, yielding a sensitivity of 95%, specificity of 42%, accuracy of 87%, and AUC of 0.74.5

An additional emerging technique is dual energy spectral CT. This technique holds the promise of improved tumor characterization while minimizing radiation exposure, decreasing scan time, and lowering the dose of iodine contrast required.6
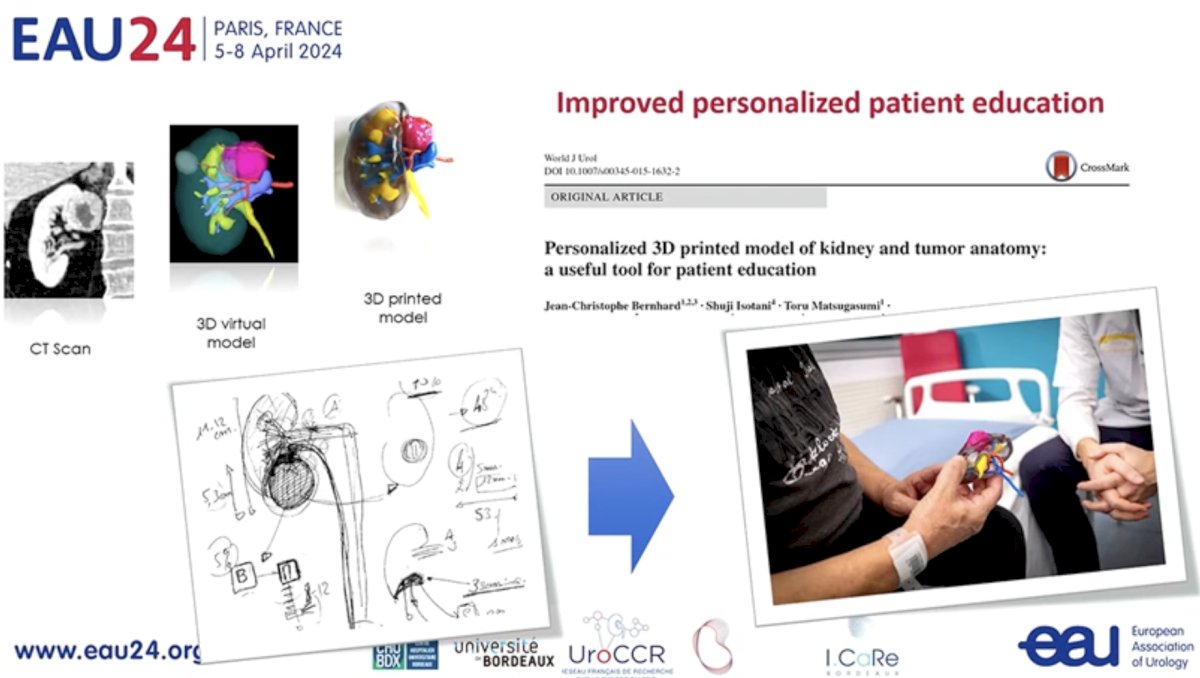
Another exciting aspect of conventional imaging is its potential to improve surgical planning. The CT-based nephrometry score objectifies the anatomical complexity of renal masses, assisting in surgical decision making, and facilitating outcome assessment. Artificial intelligence algorithms for automatic structure recognition and CT scan segmentation are being developed to improve surgical planning.
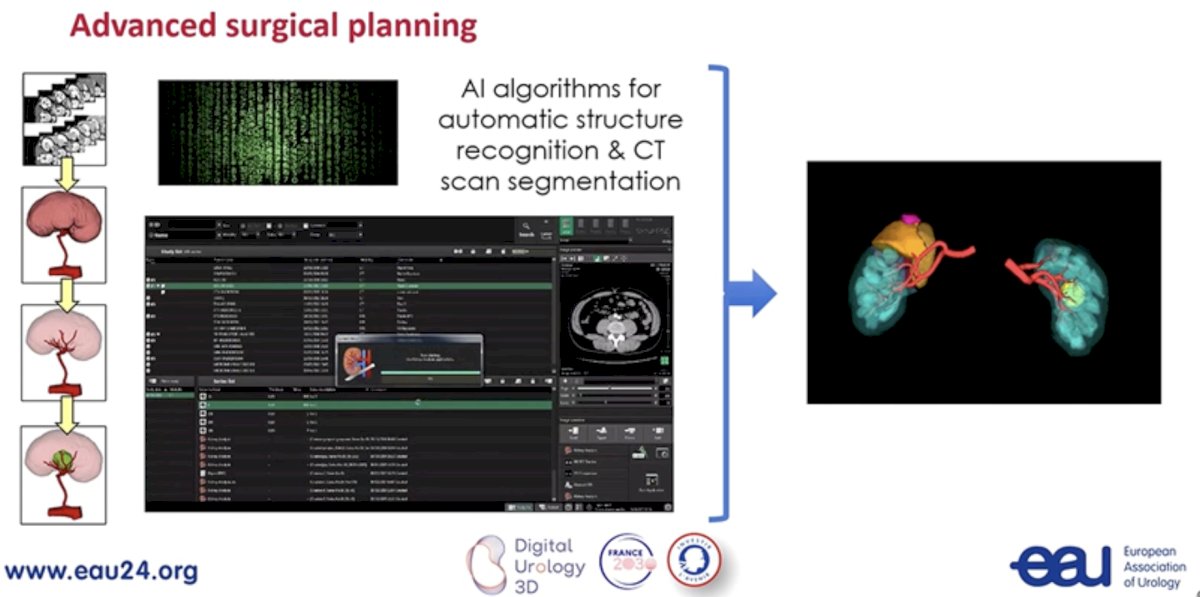
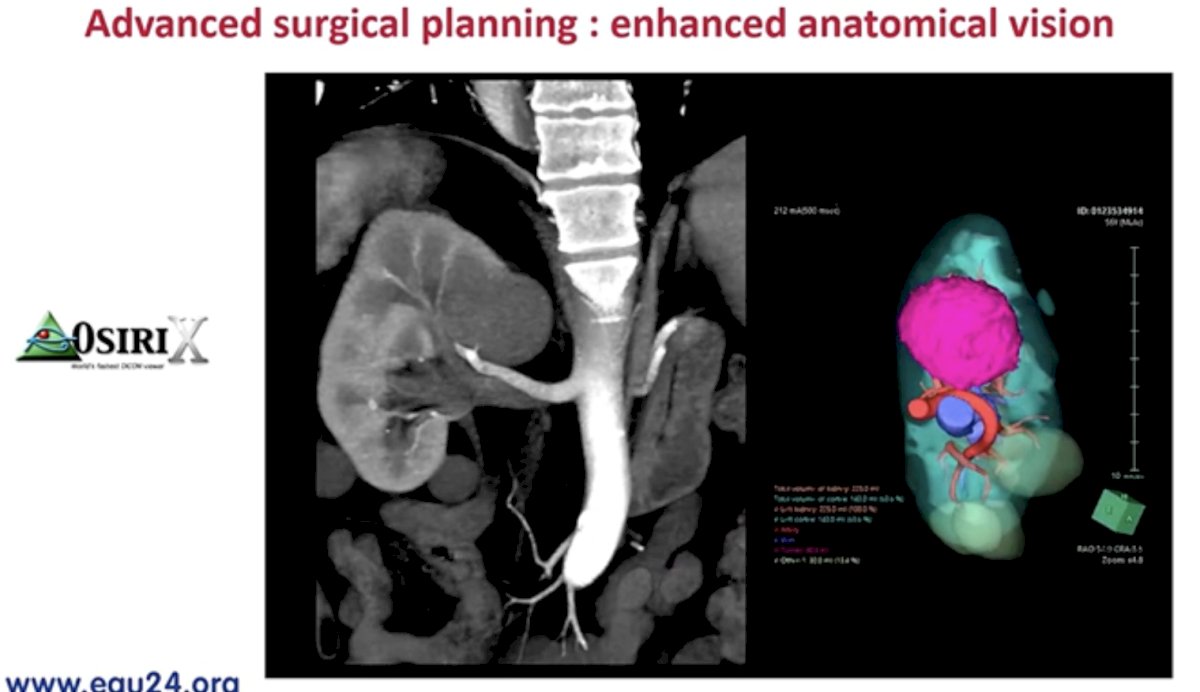
Outputs from these models are incorporated into 3D model-assisted surgeries allowing for enhanced intra-operative navigation.
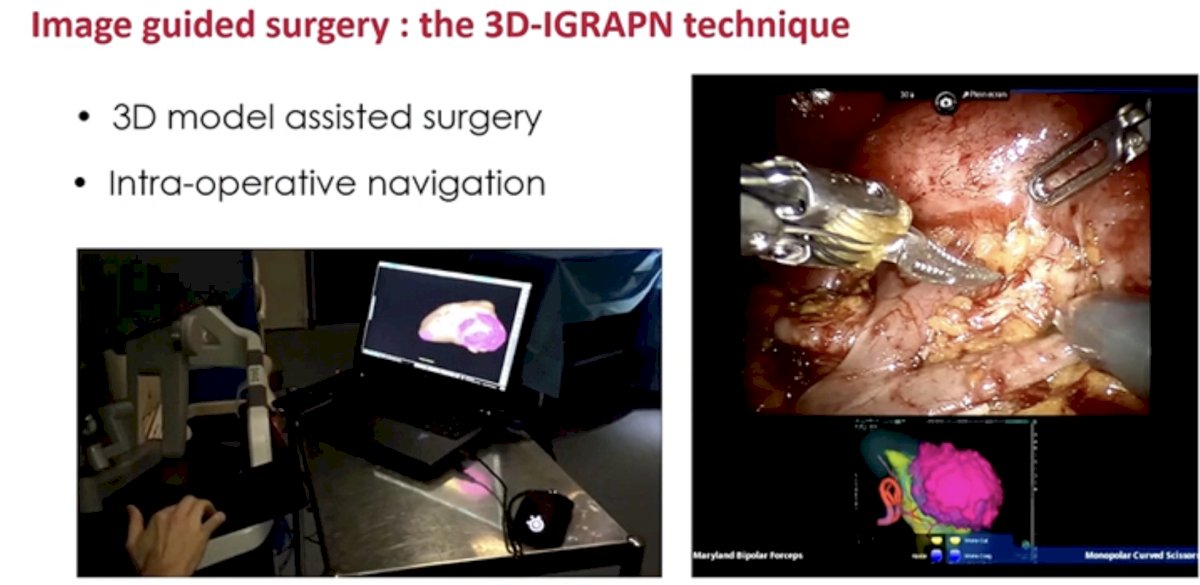
These model outputs may additionally allow for improved, personalized patient education via 3D printed models of kidney and tumor anatomy.
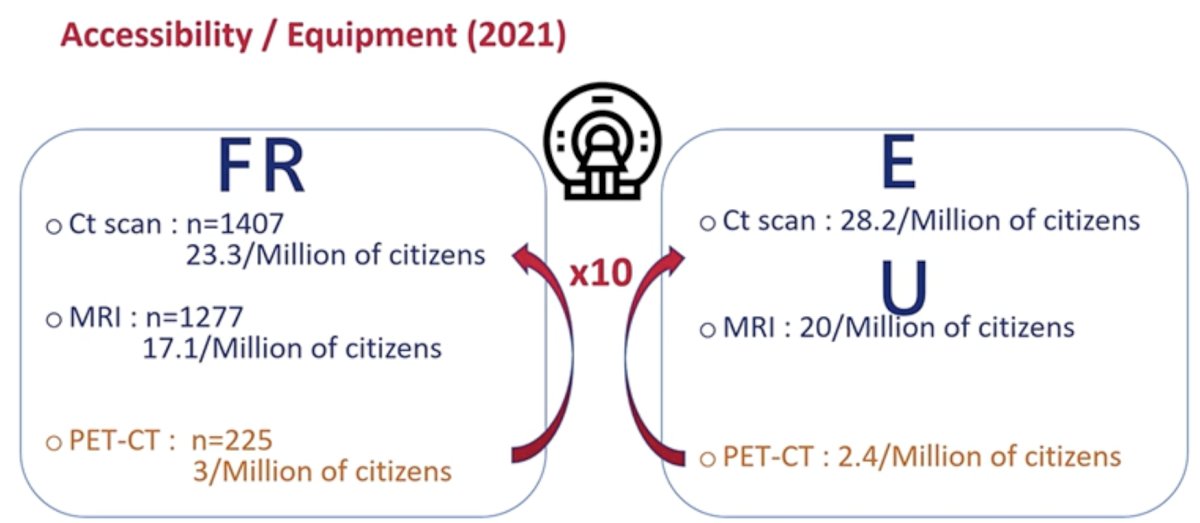
With regards to functional nuclear medicine scans, such as PET-CT, while there are clear advantages for their utilization in this space, the major limitation to their widespread use remains limitations with accessibility/equipment availability. In Europe, there are only 2.4 PET/CTs per 1 million citizens, compared to 28.2 and 20 CT scans and MRIs, respectively, per a million citizens.
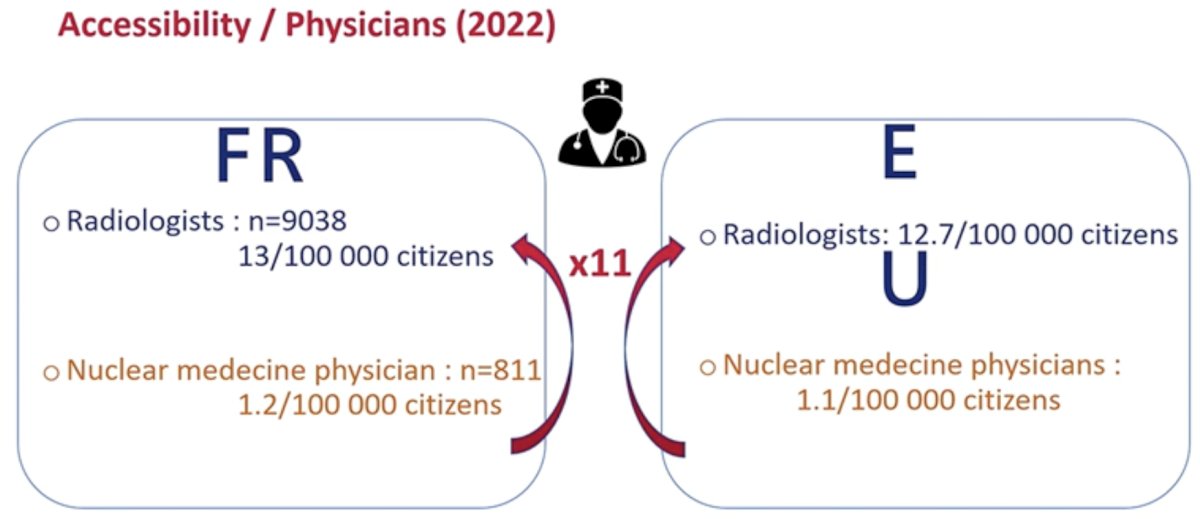
Similarly, the number of nuclear medicine physicians pales in comparison to radiologists, and this creates real world limitations to the widespread use and interpretation of such scan findings.
Professor Bernhard concluded that:
- Conventional imaging is:
- Accessible
- Efficient for RCC detection
- Reliable for staging
- Available techniques complement each other
- Shortcomings of conventional imaging can be overcome using:
- Technical improvements
- Artificial intelligence and multi-omics
- Conventional imaging ‘kills two birds with one stone’ with applications beyond staging, including outcomes prediction, surgical planning, live surgical guidance, and patient education.
Presented by: Professor Jean-Christophe Bernhard, MD, PhD, Department of Urology, Université de Bordeaux, Bordeaux, France
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024
References:- Vogel C, Ziegelmuller B, Ljungberg B, et al. Imaging in Suspected Renal-Cell Carcinoma: Systematic Review. Clin Genitourin Cancer. 2019;17(2): e345-e355.
- Boulenger de Hauteclocque A, Ferrer L, Ambrosetti D, et al. Machine-learning approach for prediction of pT3a upstaging and outcomes of localized renal cell carcinoma (UroCCR-15). BJU Int. 2023;132(2):160-169.
- Coy H, Hsieh K, Wu W, et al. Deep learning and radiomics: the utility of Google TensorFlow™ Inception in classifying clear cell renal cell carcinoma and oncocytoma on multiphasic CT. Abdom Radiol (NY). 2019;44(6):2009-2020.
- Zhang G, Shi B, Xue H, et al. Can quantitative CT texture analysis be used to differentiate subtypes of renal cell carcinoma? Clin Radiol. 2019;74(4):287-294.
- Garnier C, Ferrer L, Vargas J, et al. A CT-Based Clinical, Radiological and Radiomic Machine Learning Model for Predicting Malignancy of Solid Renal Tumors (UroCCR-75). Diagnostics (Basel). 2023;13(15):2548.
- Wei J, Zhao J, Zhang X, et al. Analysis of dual energy spectral CT and pathological grading of clear cell renal cell carcinoma (ccRCC). PLoS One. 2018;13(5):e0195699.


