(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a kidney cancer rapid fire debate, and a presentation by Dr. Thomas Powles discussing that we should be treating all pT3a grade IV/N0 patients after nephrectomy with adjuvant immunotherapy.
The context of this discussion is based on a case of a male patient born in 1948 who presented in October 2021 with persistent gross hematuria and subsequent CT imaging showing a tumor in the left kidney, including the renal pelvis. Ureteroscopy + biopsy demonstrated a solid tumor in the low calix, histology showing necrotic material. An MRI ultimately showed an infiltrative lower pole tumor. In December 2021, he underwent a left nephroureterectomy with pathology demonstrating clear cell renal cell carcinoma, pT3aN0 (0/3 lymph nodes), grade IV.
Dr. Powles notes that immune checkpoint inhibition is associated with cure in advanced RCC and earlier interventions are likely to be better. In the KEYNOTE-564 study,1 patients were ≥18 years of age and had histologically confirmed clear cell RCC with or without sarcomatoid features, increased risk of recurrence, ECOG performance status of 0 or 1, nephrectomy and/or metastasectomy ≤12 weeks before randomization, and had no prior systemic therapy for RCC. Patients were randomly allocated 1:1 to receive pembrolizumab 200 mg or placebo intravenously every 3 weeks for ≥17 cycles (~1 year) or until disease recurrence, intolerable toxicity, or withdrawal of consent: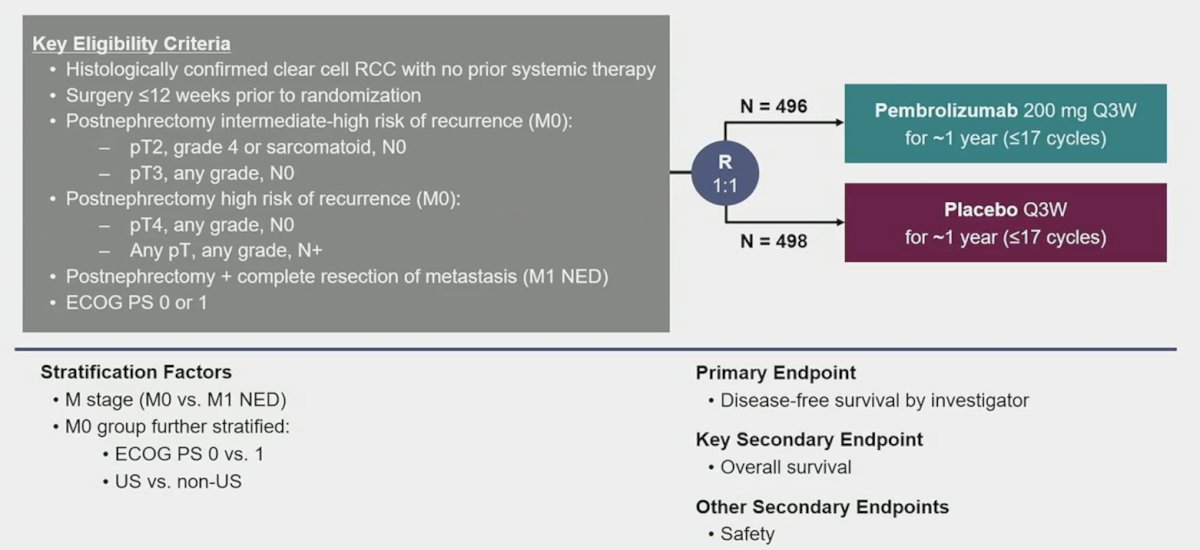
There was a statistically significant improvement in overall survival observed with pembrolizumab vs placebo (medians not reached, HR 0.62, 95% CI 0.44−0.87; p = 0.0024):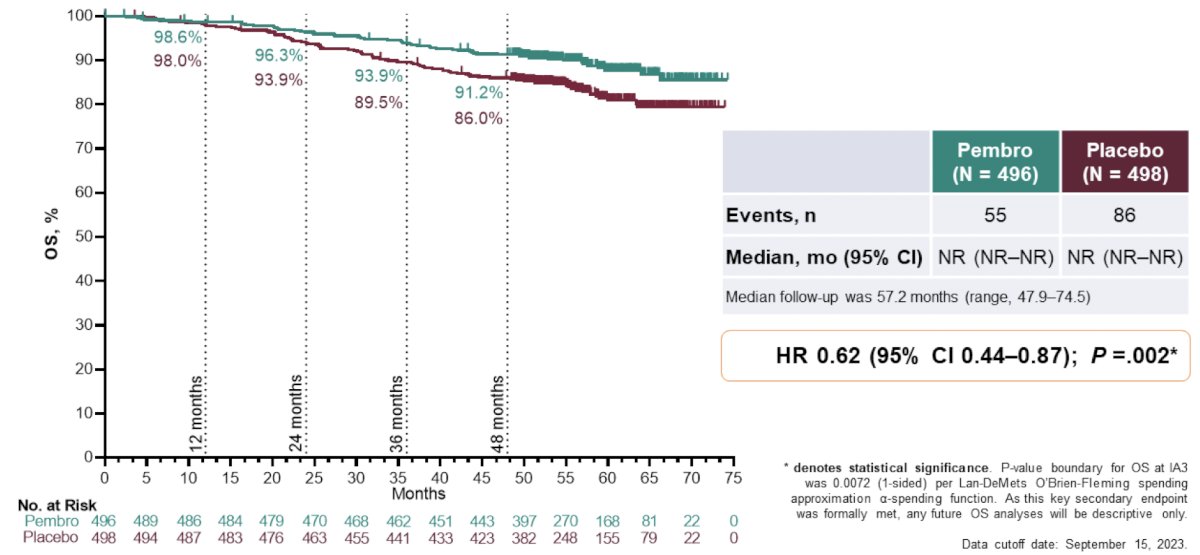
A total of 55 overall survival events were observed in the pembrolizumab arm and 86 in the placebo arm. The estimated overall survival rate at 48 months was 91.2% with pembrolizumab and 86.0% with placebo. Dr. Powles notes that it is important for us to counsel our patients on not just the 38% reduction in mortality, but also that the absolute benefit with 1 year of adjuvant pembrolizumab is ~5-10%. Encouragingly, the overall survival benefit was observed across key subgroups, including in patients with:
- M0 disease: HR 0.63, 95% CI 0.44−0.90
- M1 no evidence of disease: HR 0.51, 95% CI 0.15−1.75
- PD-L1 CPS <1: HR 0.65, 95% CI 0.31−1.38
- PD-L1 CPS ≥1: HR 0.62, 95% CI 0.42−0.91
- Presence of sarcomatoid features: HR 0.69, 95% CI 0.28−1.70
- Absence of sarcomatoid features: HR 0.57, 95% CI 0.39−0.84
Moreover, the observed disease-free survival benefit with pembrolizumab vs placebo was consistent with prior interim analyses (HR 0.72; 95% CI 0.59−0.87):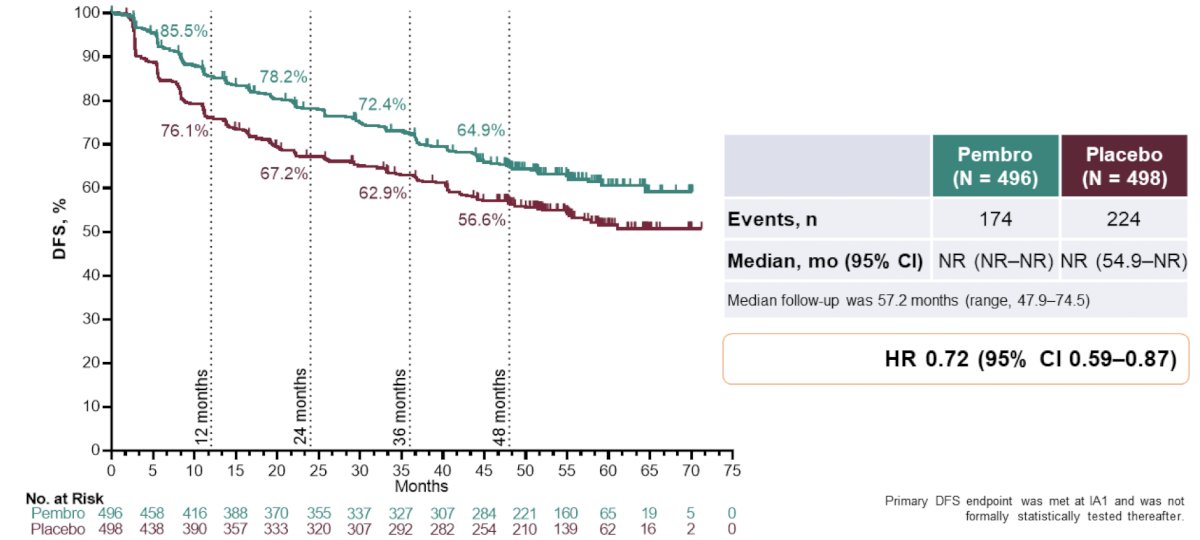
With regards to subsequent therapies in the intention to treat population, 79.5% of patients in the pembrolizumab arm versus 81.4% of patients in the placebo arm had subsequent therapy, most commonly another systemic anti-cancer drug therapy:
Dr. Powles notes that if we take six different patients in their kidney cancer journey, there may be several different iterations of when they are receiving local therapy, immune therapy, and VEGF therapy, all of which may be reasonable. Thus, no one size necessarily fits all for this patient cohort: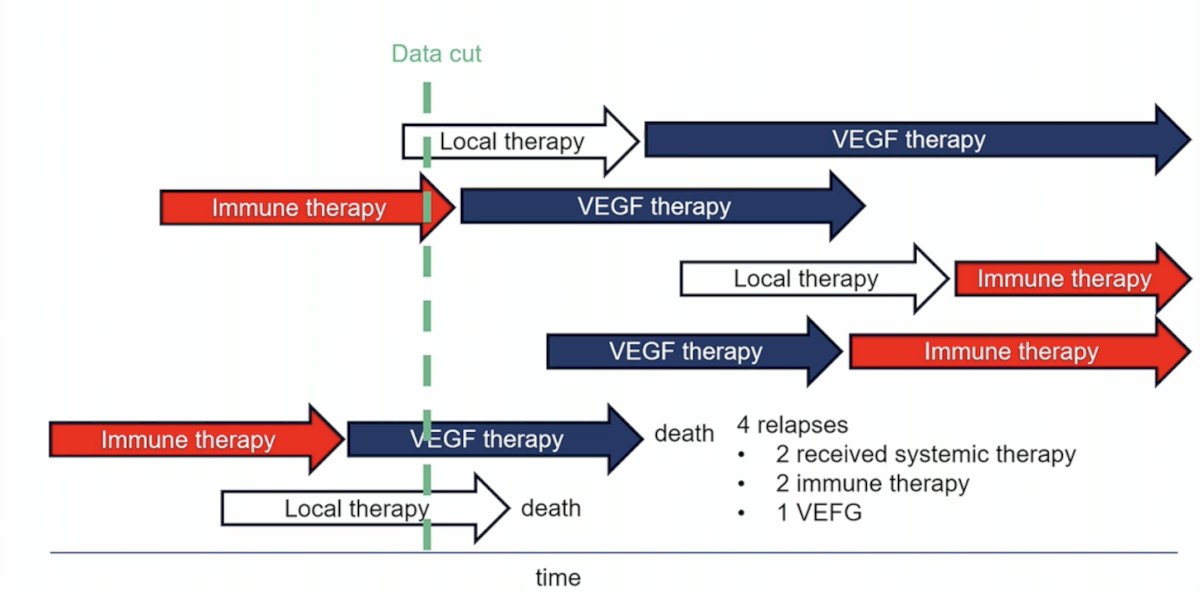
Admittedly, Dr. Powles is perplexed regarding why the nivolumab + ipilimumab vs placebo and nivolumab vs placebo adjuvant therapy CheckMate 914 trial was negative, noting quite similar trial designs with KEYNOTE-564 other than duration of therapy (nivolumab – 6 months; pembrolizumab – 12 months):2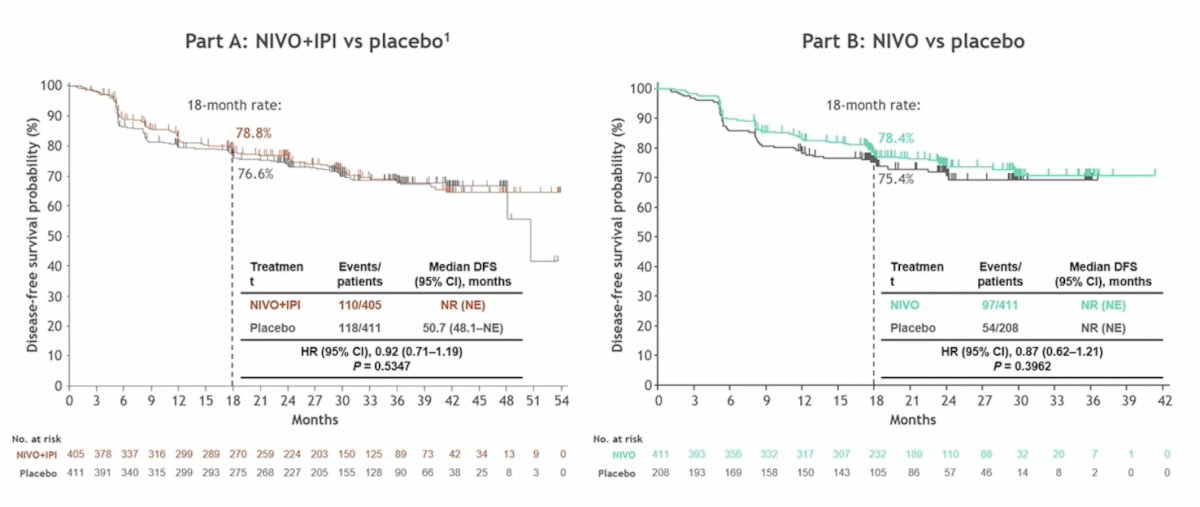
Dr. Powles concluded his presentation discussing that we should be treating all pT3a grade IV/N0 patients after nephrectomy with adjuvant immunotherapy with the following statements:
- Overall survival signals in adjuvant renal cancers have been elusive
- However, adjuvant pembrolizumab has broken this spell of silence
- Patients can be recommended this therapy with some caveats including toxicity
- In this case, T3 and G4 are still considered intermediate risk disease (30% risk of relapse). Biomarkers are needed to determine which patients need therapy
- Issues around the negative nivolumab data are unclear but may be due to duration of therapy
Presented by: Professor Thomas Powles, Barts Cancer Institute, London, UK
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021 Aug 19;385(8):683-694.
- Motzer RJ, Russo P, Grunwald V, et al. Adjuvant nivolumab plus ipilimumab versus placebo for localized renal cell carcinoma after nephrectomy (CheckMate 914): A double-blind, randomized, phase 3 trial. Lancet. 2023 Mar 11;401(10379):821-832.


