(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Professor Henk van der Poel discussed why patients with prostate-specific membrane antigen (PSMA)-detected M1a disease should be treated similarly to patients with conventional imaging-detected M0 disease.
M1a prostate cancer is defined by the presence of non-regional lymph nodes present above the bifurcation of the common iliac arteries, in the absence of other metastases, although whether inguinal and/or pararectal disease should be considered M1a disease remains unclear. Between 2010 and 2018, the age‐adjusted incidence of de novo M1a prostate cancer in The Netherlands increased from 0.47 to 1.89 cases per 100,000 population (imaging modality was unspecified).1
Professor van der Poel noted that the current EAU guidelines strongly recommend offering a combined systemic + local therapy approach with ADT + non-curative prostate radiotherapy for patients with M1a disease. However, these recommendations are based on evidence from the pre-PSMA-PET/CT era, and he argued that patients with PSMA-detected M1a disease should be considered different.
It has long been established that patients with non-regional nodal limited metastatic disease have superior survival outcomes compared to those with bone and visceral metastases.2 And the prevalence of such metastases is more common than originally thought. A recent systematic review published in 2023 by the EAU Young Academic Urologists Prostate Cancer Working Party demonstrated that 1–16% of high-risk prostate cancer patients referred for PSMA-PET imaging have evidence of M1a disease, which accounts for 42% of all metastases detected in such patients.3
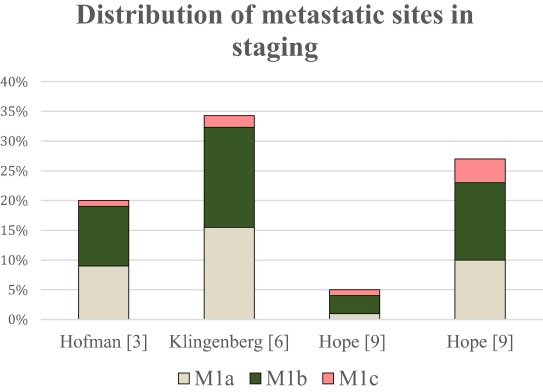
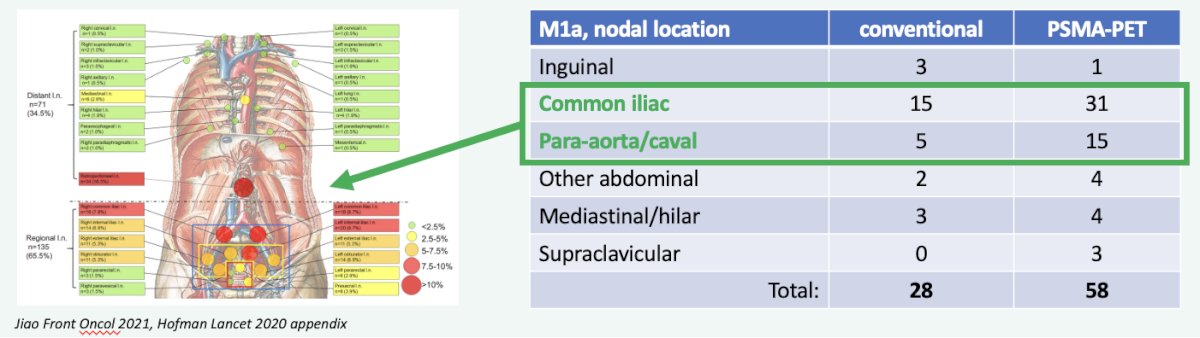
Additional evidence supporting the higher than previously recognized prevalence of such M1a metastases comes from the proPSMA study, which was a multi-center, two-arm randomized controlled trial of prostate cancer patients considered for curative intent radical prostatectomy or radiotherapy. Eligible patients had at least one high-risk factor including PSA ≥ 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging consisting of bone scan and CT or 68Ga-PSMA-11 PET/CT. In this trial, M1a disease was detected more than twice as frequently (58 versus 28) with PSMA-PET/CT.4
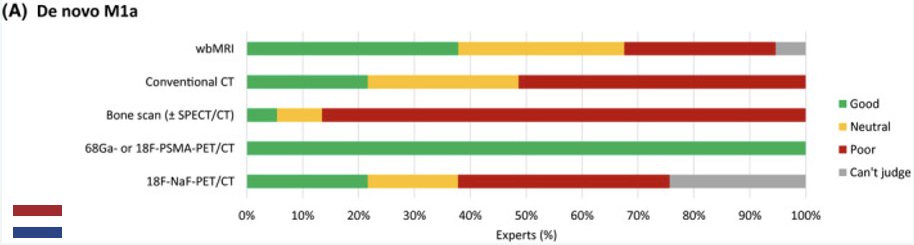
Given the improved performance characteristics of PSMA-PET/CT for detecting M1a disease, it is now unanimously considered the optimal imaging modality of choice for staging de novo M1a patients in a survey of Dutch experts. Additionally, the report from the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) demonstrated that 77% of experts recommend PSMA PET for the majority of patients with clinically localized, high-risk prostate cancer.5
Professor van der Poel highlighted that one of the main limitations of the current literature for systemic therapy in the metastatic hormone-sensitive setting is the limited number of M1a patients included. As summarized in the figure below, the majority of the seminal phase 3 trials have included either none or a limited number of M1a patients.
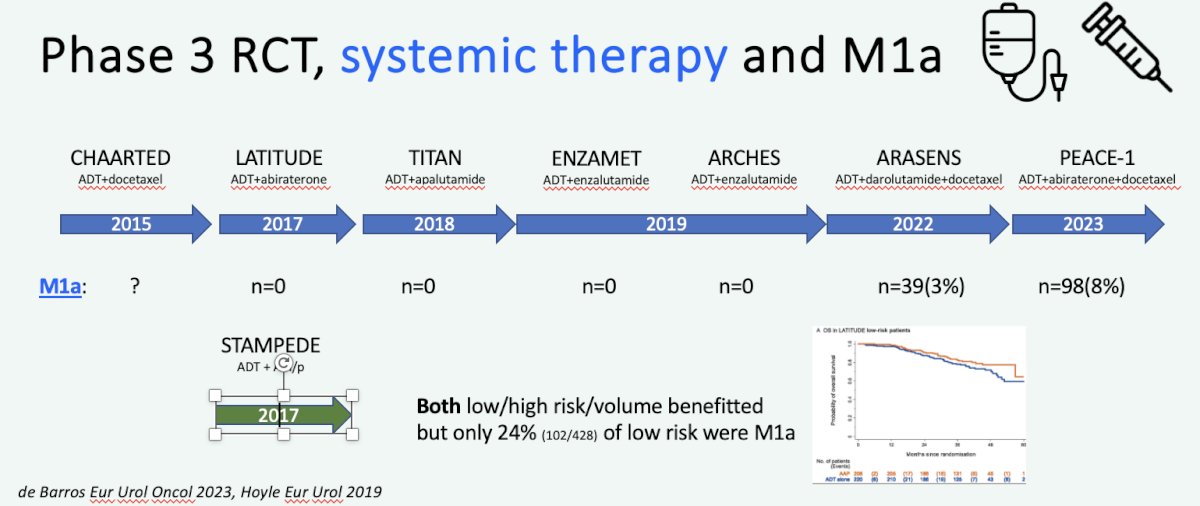
This is also the case for the recently presented PEACE-1 radiotherapy data among patients with low volume metastatic disease. While there was evidence of a radiographic progression-free survival benefit for prostate radiotherapy in low-volume metastatic prostate cancer patients receiving abiraterone plus standard of care therapy (median 7.5 versus 4.4 years, p=0.02), there was no data available specifically for the M1a patients.
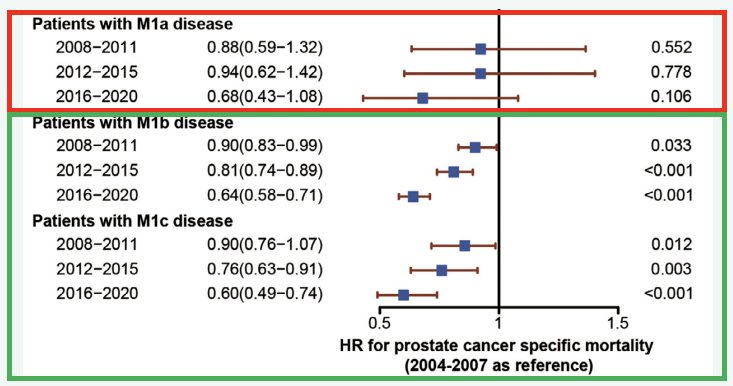
Another worrying trend is that while survival for M1 prostate cancer patients has overall improved over the last decade, it does not appear that this trend holds true for patients with M1a disease. Is this a result of us under-treating M1a patients?
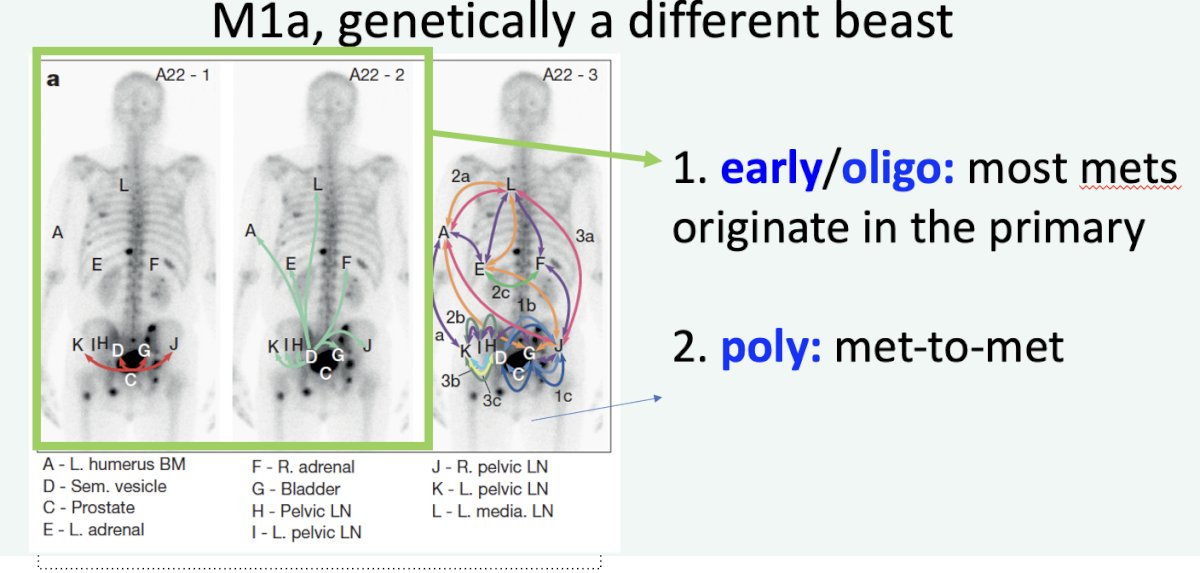
Another important consideration when treating M1a patients is to recognize that M1a disease is a genetically distinct entity. In patients with early/oligometastatic disease, most metastases originate from the primary site. Conversely, among patients with polymetastatic disease, spread from one metastatic site to the other is much more common.6 This provides support for locally treating M1a patients.
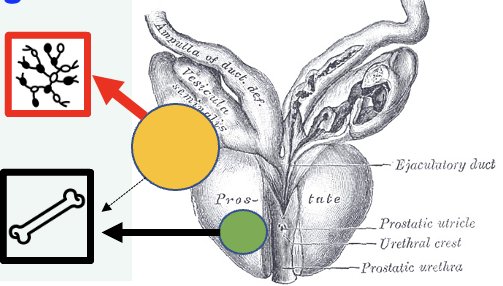
Phylogenetic analysis has additionally demonstrated that nodal metastases may be a distinct entity compared to bone metastases, whereby lymph node metastases may not be an intermediate developmental step for distant osseous metastases, but rather represent a distinct metastatic lineage.7
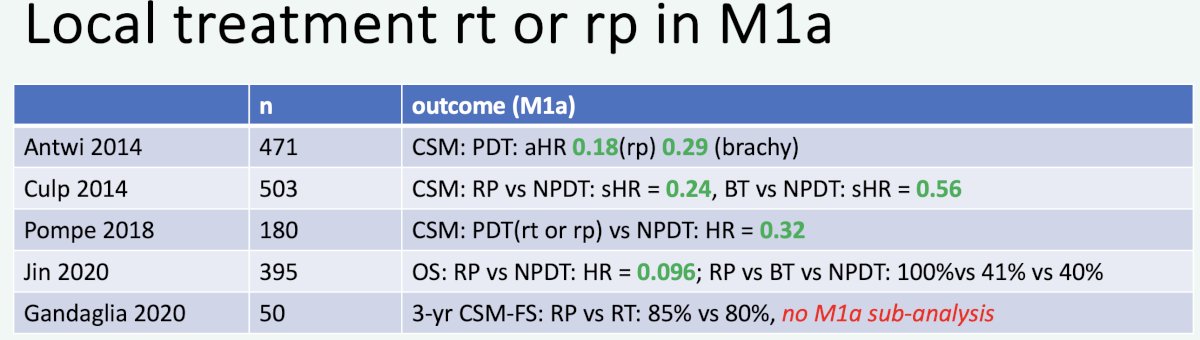
In 2023, Professor van der Poel’s group published the results of a systematic review of local and/or metastasis-directed therapy in patients with hormone-sensitive M1a prostate cancer. Overall, the group concluded that “ in de novo M1a prostate cancer patients, prostate-directed therapy was associated with improved oncological outcomes compared with no prostate-directed therapy”.8 Summarized below are the retrospective studies evaluating prostate-directed therapy for M1a patients:
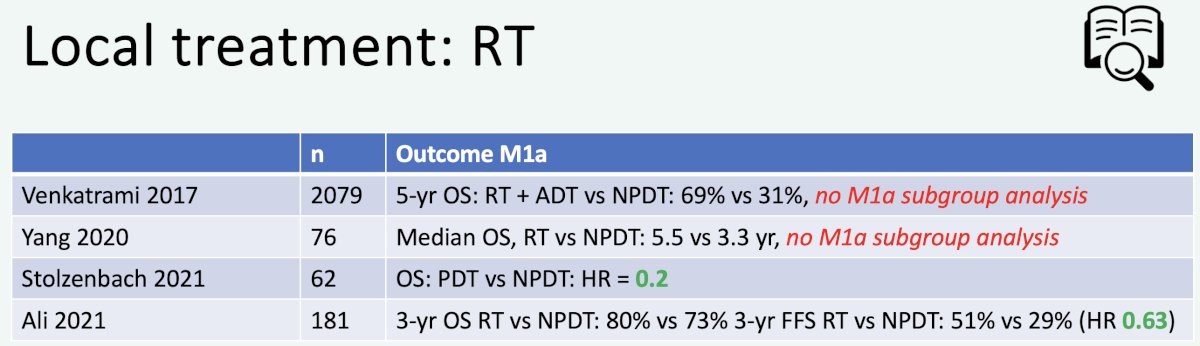
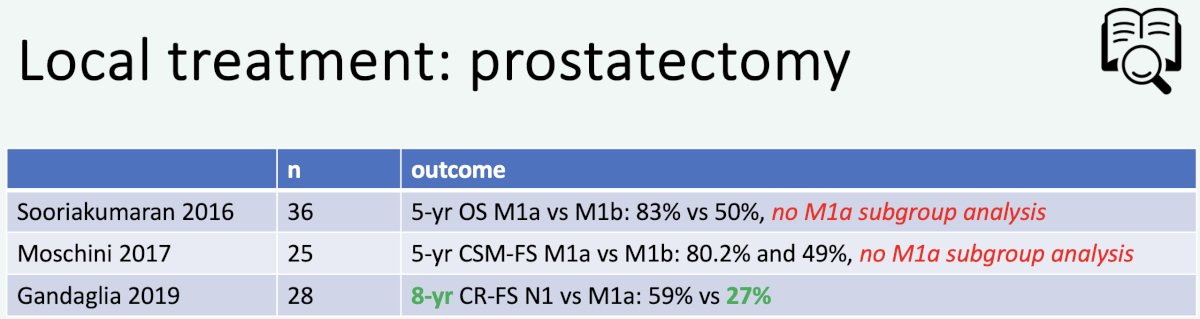
What is the specific evidence supporting local therapy for M1a patients? In 2016, Gandaglia et al. demonstrated that while outcomes post-surgery are worse for patients with evidence of M1a disease, compared to those with N1 disease, the 8-year clinical recurrence-free survival was 41% for such patients undergoing surgery.
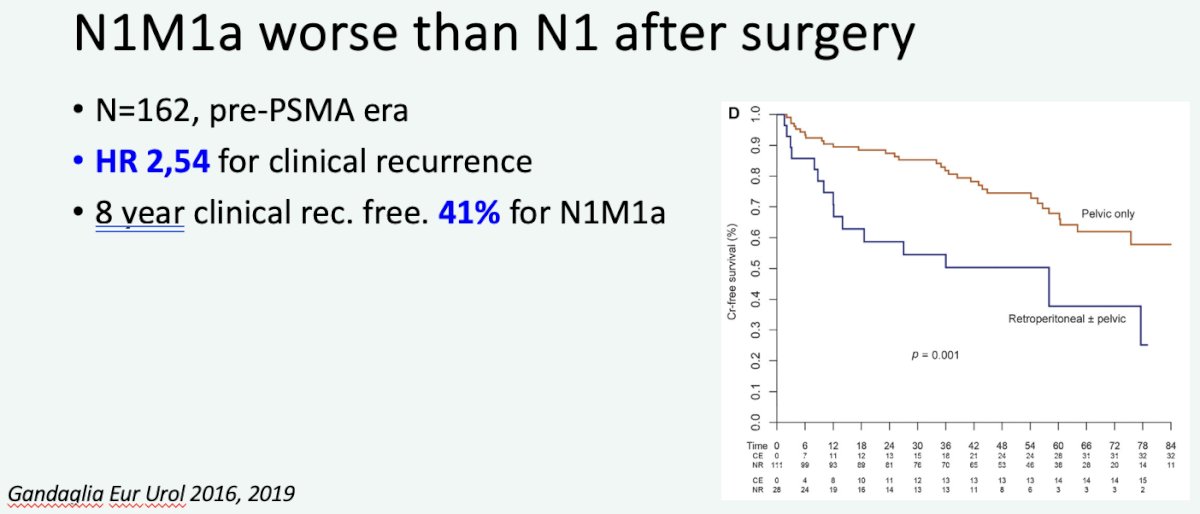
Additionally, it appears that M1a (common iliac node positive) patients fare similarly to those with N1 disease when treated with radiotherapy.9
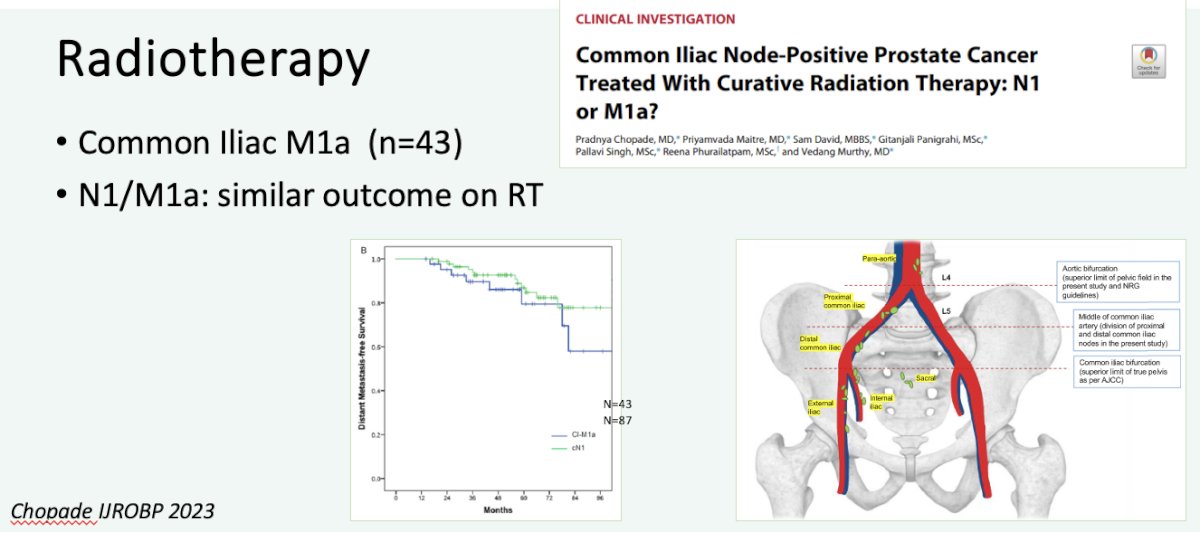
The STAMPEDE Arm H trial has demonstrated that prostate radiotherapy improved overall survival in patients with low volume metastatic disease (HR 0.64, 95% CI 0.52-0.79).10 Subgroup analysis of the ‘only non-regional lymph node metastases’ (i.e., M1a) subgroup of 181 patients (9% of total cohort) has demonstrated that prostate radiotherapy addition to standard of care therapy improves both overall (HR: 0.60) and failure-free survivals (HR: 0.63).
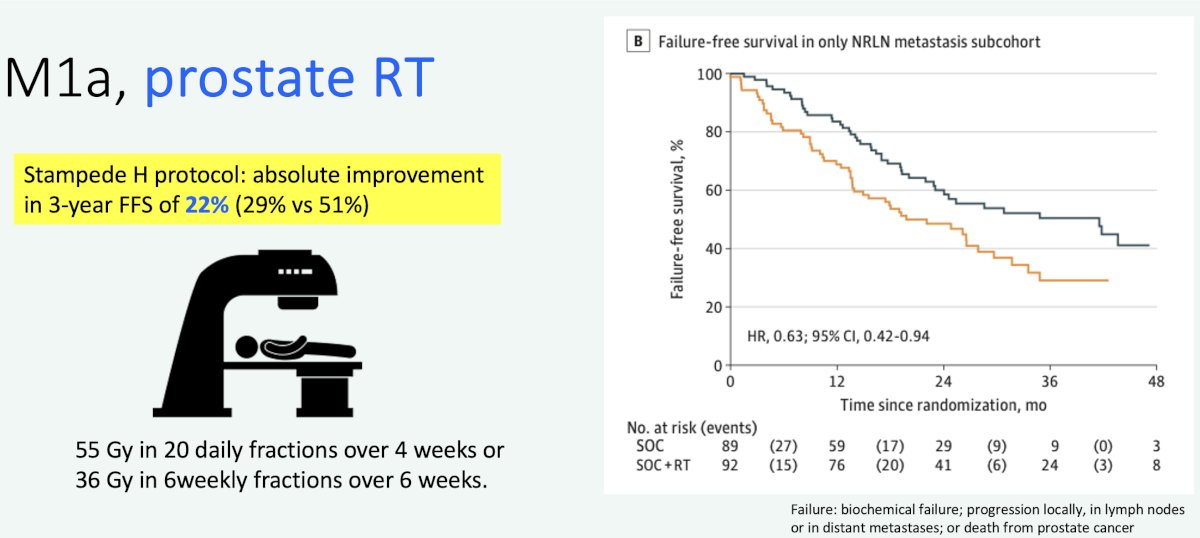
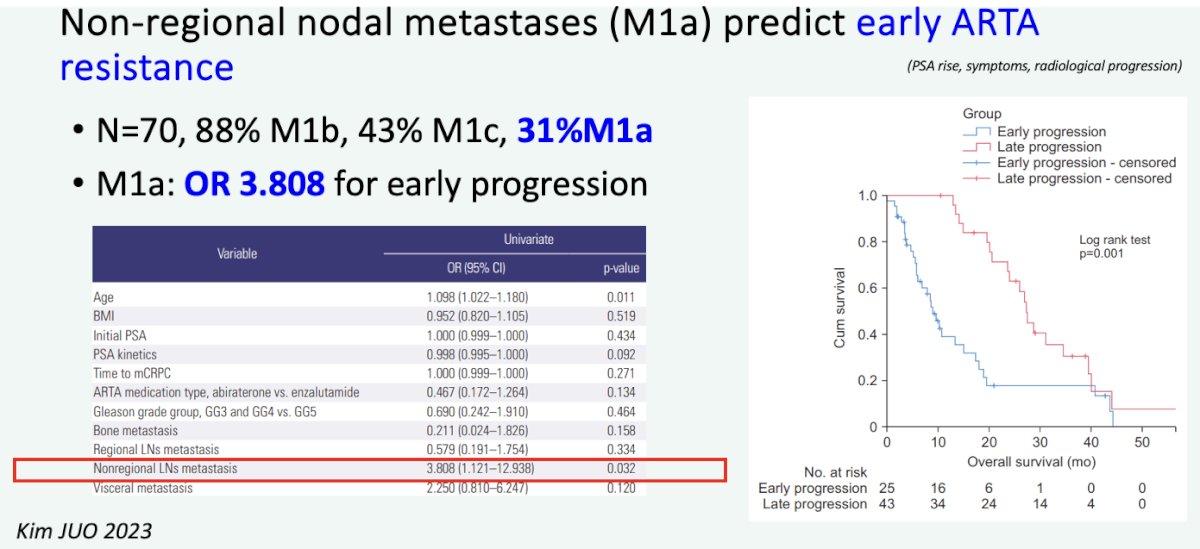
Professor van der Poel highlighted that an additional reason to consider prostate-directed local therapy is to avoid the toxicities that emerge with ADT. Additionally, there is evidence to suggest that patients with M1a disease may develop early resistance to androgen receptor pathway inhibitors.
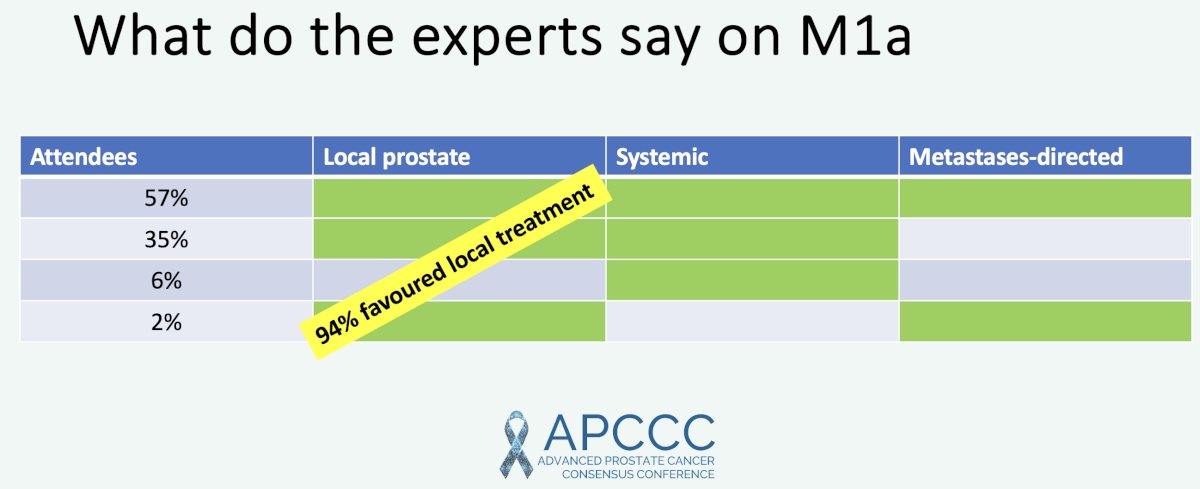
What do the experts say about M1a disease? The 2022 APCCC report demonstrated that 95% of prostate cancer experts favored local treatment for M1a disease.5
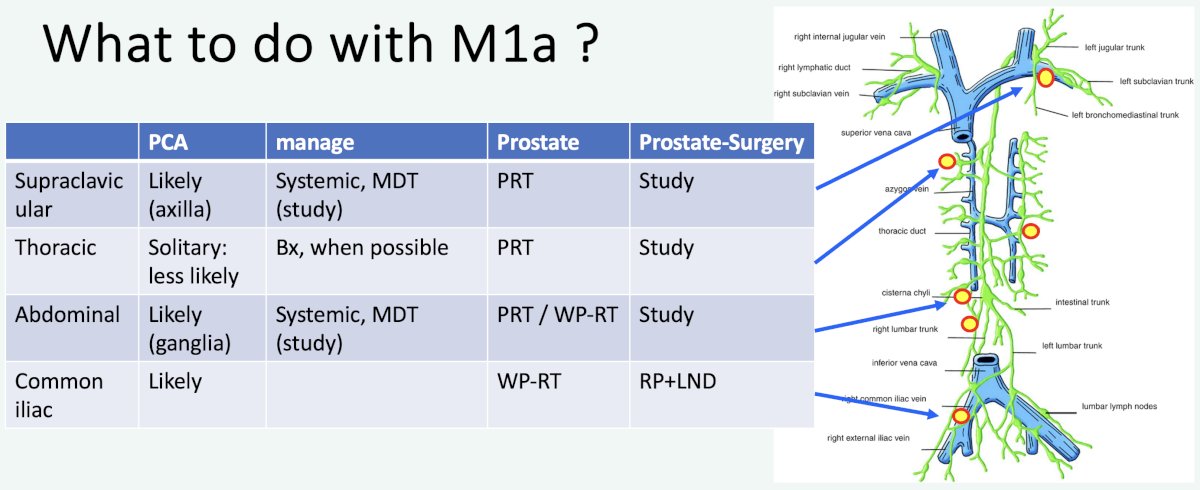
So what should one do with M1a patients? Professor van der Poel proposed the following approach based on the location of M1a disease:
He concluded his presentation by highlighting that M1a prostate cancer remains:
- An understudied and heterogenous disease
- Increasingly more commonly recognized (~40% of all metastases on PSMA PET for high-risk disease)
- Increasingly being found at lower disease volumes with PSMA PET
- Amenable to non-systemic treatment options, including radiotherapy and metastasis-directed therapy
Presented by: Professor Henk G. van der Poel, MD, PhD, Department of Urology, Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, The Netherlands
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024References:
- Aluwini S, Prea-Lager DE, de Barros H, et al. M1a prostate cancer: Results of a Dutch multidisciplinary consensus meeting. BJUI Compass. 2021;2(3): 159-168.
- Heesterman BL, van der Poel HG, Schoots IG, Mehra N, Aben KKH. Prognostic importance of concomitant non-regional lymph node and bone metastases in men with newly diagnosed metastatic prostate cancer. BJU Int. 2022;130(2): 217-225.
- Mattana F, Muraglia L, Rajwa P, et al. Metastatic Sites' Location and Impact on Patient Management After the Introduction of Prostate-specific Membrane Antigen Positron Emission Tomography in Newly Diagnosed and Biochemically Recurrent Prostate Cancer: A Critical Review. Eur Urol Oncol. 2023;6(2): 128-136.
- Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020 Apr 11;395(10231):1208-1216.
- Gillessen S, Bossi A, Davis ID, et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer. 2023;185: 178-215.
- Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520(7547): 353-357.
- Mangiola S, Hong MKH, Cmero M, et al. Comparing nodal versus bony metastatic spread using tumour phylogenies. Sci Rep. 2016;6:33918.
- de Barros HA, van Beurden I, Droghetti M, et al. Role of Local and/or Metastasis-directed Therapy in Patients with Hormone-sensitive M1a Prostate Cancer-A Systematic Review. Eur Urol Oncol. 2023;6(1): 16-27.
- Chopade P, Maitre P, David S, et al. Common Iliac Node-Positive Prostate Cancer Treated With Curative Radiation Therapy: N1 or M1a? Int J Radiat Oncol Biol Phys. 2022;114(4): 711-717.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Medicine. 2022;19(6):e1003998.
- Ali A, Hoyle A, Haran AM, et al. Association of Bone Metastatic Burden With Survival Benefit From Prostate Radiotherapy in Patients With Newly Diagnosed Metastatic Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2021;7(4): 555-563.


