(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Dr. Christopher Sweeney discussed why patients with prostate-specific membrane antigen (PSMA)-detected M1a disease should be treated using a similar approach to those with ‘classic’ metastatic disease on conventional imaging.
Dr. Sweeney began with a case presentation of a 68-year-old man with 6 months of increasing nocturia and urinary frequency. A digital rectal examination confirmed the presence of a clear, definite nodule. Serum PSA level at that time was 45 ng/ml. An MRI demonstrated evidence of T3 disease, and a biopsy showed evidence of Gleason Score 8 disease in 4 of 12 sampled cores. A PSMA-PET/CT was obtained and demonstrated the following:
- 5 retroperitoneal lymph nodes all <1 cm
- No evidence of bone or visceral metastases
What is the optimal treatment plan in this patient? To date, there is no study to directly address this specific question; however, data from the STAMPEDE-Abiraterone and radiotherapy PEACE-1 data presented at ASCO 2023 can be used to support the argument for intensified systemic therapy for PSMA-detected M1a disease.
The STAMPEDE-abiraterone trial included prostate cancer patients with high-risk features, which included node-positive disease or, if node negative, then ≥2 of the following: cT3–4, Gleason sum score of 8–10, and serum PSA ≥40 ng/mL (trial also included patients with relapsing disease and high-risk features). Patients were randomized to receive prostate radiotherapy + 3 years of ADT +/- 2 years of abiraterone acetate/prednisone. The addition of abiraterone in this setting significantly improved overall survival in this cohort of patients with high-risk, localized disease (HR: 0.60, 95% CI: 0.48 –0.73, p<0.0001).1 Notably, all patients in this trial had localized disease (i.e., ‘M0’) per conventional imaging with CT A/P and bone scan. Dr. Sweeney argued that many of these patients are likely to have had evidence of underlying M1a disease had they undergone a PSMA scan, and thus the results of this trial provide support for systemic therapy intensification for such patients given the likelihood of this scenario.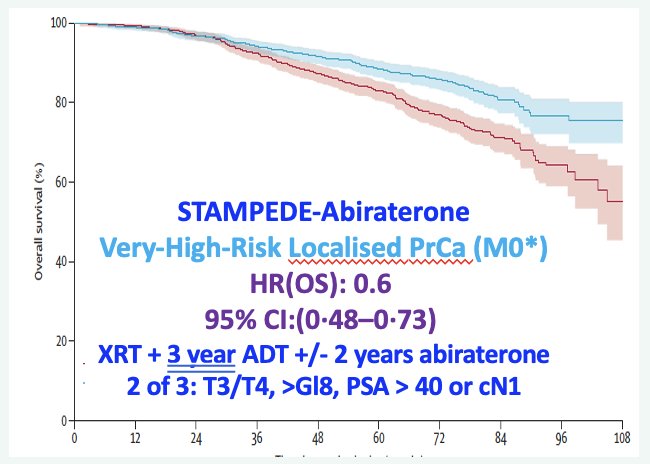
Next, Dr. Sweeney discussed the PEACE-1 radiotherapy data presented by Dr. Bossi at ASCO 2023. In this trial, patients with conventional imaging-defined CHAARTED low-volume metastatic hormone-sensitive disease were randomized to receive either standard of care therapy (ADT +/- docetaxel) or standard of care therapy + abiraterone. Furthermore, given the 2x2 factorial design of this trial, these patients were further randomized to receive prostate radiotherapy versus no local therapy. There was evidence of a radiographic progression-free survival (rPFS) benefit with prostate radiotherapy only in those patients who received concurrent abiraterone (median rPFS: 7.5 versus 4.4 years, p=0.02). These results highlight the importance of treatment intensification with an androgen receptor pathway inhibitor for such patients, with abiraterone acting as an ‘effect modifier’ in this setting. This patient population is different to that from the STAMPEDE-Abiraterone trial given that these patients had evidence of low-volume metastases on conventional imaging. Dr. Sweeney suggested that many of these patients are likely to have had evidence of more ‘diffuse M1a’ disease on PSMA-PET/CT had they been scanned. Either way, the results of both trials provide ‘indirect’ evidence to support the use of intensified systemic therapy for patients with PSMA-PET/CT-detected M1a disease, similar to that for patients with conventional imaging-detected M1 disease.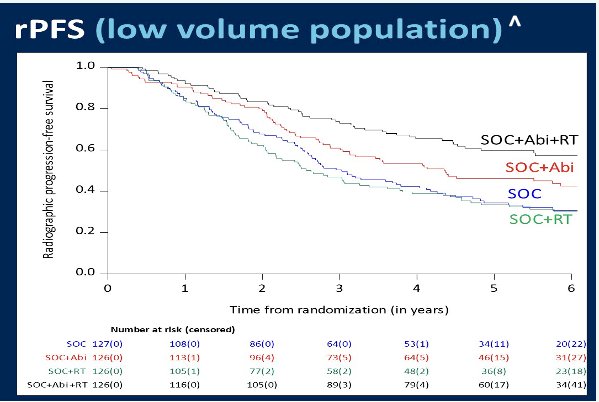
Although we currently use conventional imaging-defined findings and cut-offs to determine optimal systemic therapy approaches, Dr. Sweeney acknowledged that PSMA-PET/CT imaging is more precise/accurate, compared to conventional imaging, and that he is not proposing we ‘turn back time’ and go back to CT A/P and technetium bone scans. But what we do need is a bridging study to define the cut-points from a PSMA-PET-CT to determine who with PSMA-PET M1a disease benefits from triplet therapy with ADT + androgen receptor pathway inhibitor + prostate radiotherapy or docetaxel. When such cut-points have been defined, only then will we know the best personalized treatment plan and can ‘fire away’ with confidence when a patient says: “Doc, hit me with your best shot!”
Dr. Sweeney highlighted important differences in the information that we can obtain from conventional imaging (CT A/P and bone scan) versus PSMA-PET/CT. PSMA-PET/CT evaluates cancer directly and allows for the detection of smaller bulks of cancer. Conversely, with conventional imaging, larger disease bulks are detected (often >1 cm) and these are likely to harbor a larger number of clones with an increased potential for clone resistance. Additionally, M1a disease detected on conventional imaging is more likely to have spread to bones (present but not visualized), causing it to become more resistant, adaptable, and aggressive given its interposition within the bone milieu. These differences in toto suggest that the likelihood of disease eradication with intense systemic therapy is much more likely for PSMA-detected M1a disease and provides further support for treating these patients with such an approach. The table below highlights the current challenges in clinical practice when relying exclusively on PSMA-PET/CT to direct clinical treatment strategies for PSMA-detected M1a patients.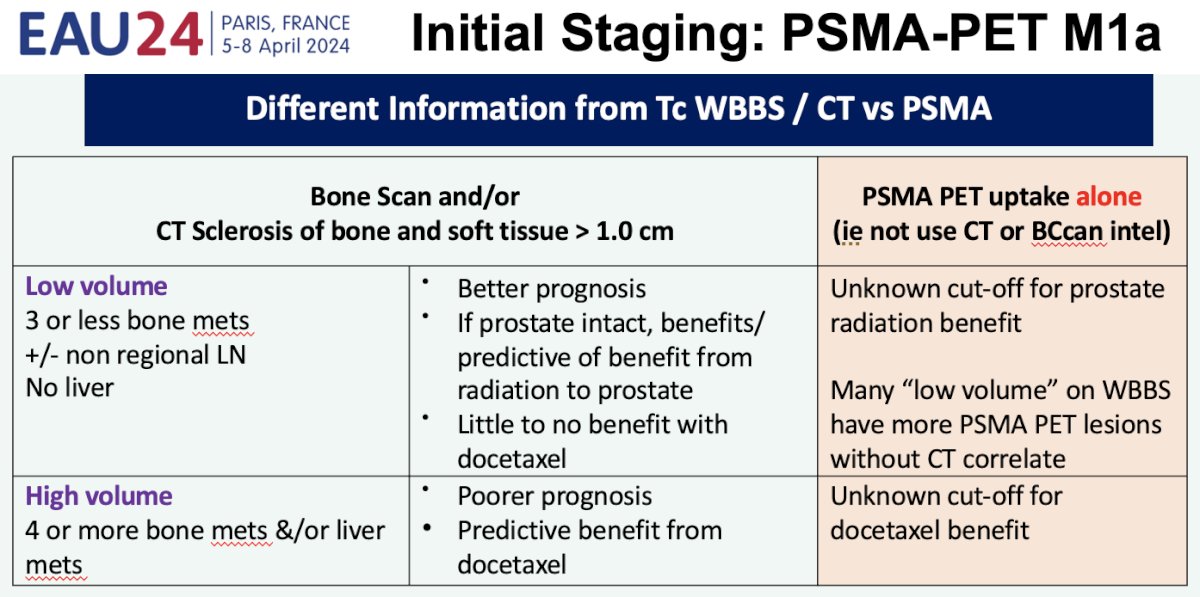
Now that Dr. Sweeney has made his ‘case’ for treating PSMA-detected M1a disease similar to conventional imaging-detected M1 disease, what specific treatment should these patients receive? He argued that a multimodal approach with combination systemic and local therapies is likely to be the answer for these PSMA M1a patients. There is cumulative evidence from trials over the last decade that combination radiotherapy plus hormonal therapy outperforms radiotherapy alone or hormone therapy alone for patients with high-risk ‘localized’ disease on conventional imaging.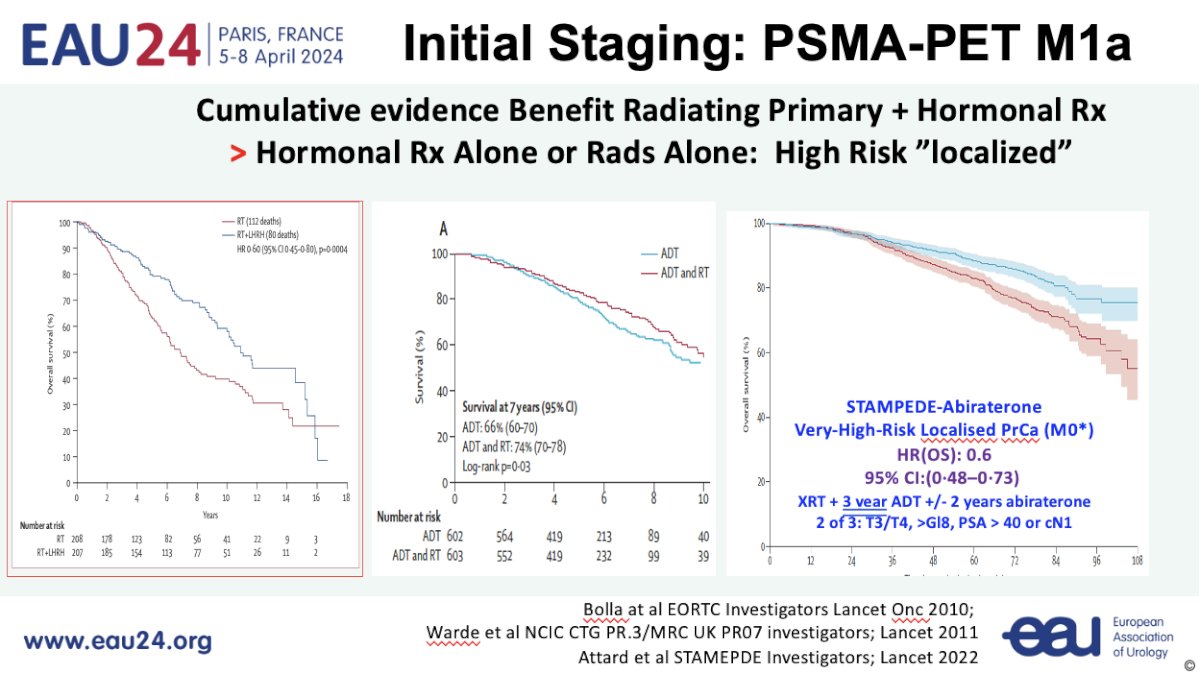
Working under this assumption of a synergistic effect for combination therapy, the first question to address is whether there is a benefit to radiotherapy in these patients. Data from HORRAD,2 STAMPEDE Arm H,3 a STOPCaP meta-analysis of HORRAD and STAMPEDE,4 and PEACE-1 all suggest that there is a survival benefit for adding prostate radiotherapy in the low-volume metastatic setting (≤5 lesions). Given that PSMA-detected M1a patients are likely to fulfill such criteria, with an even lower disease burden, Dr. Sweeney argued that it is likely safe to assume that such patients are likely to benefit from prostate radiotherapy.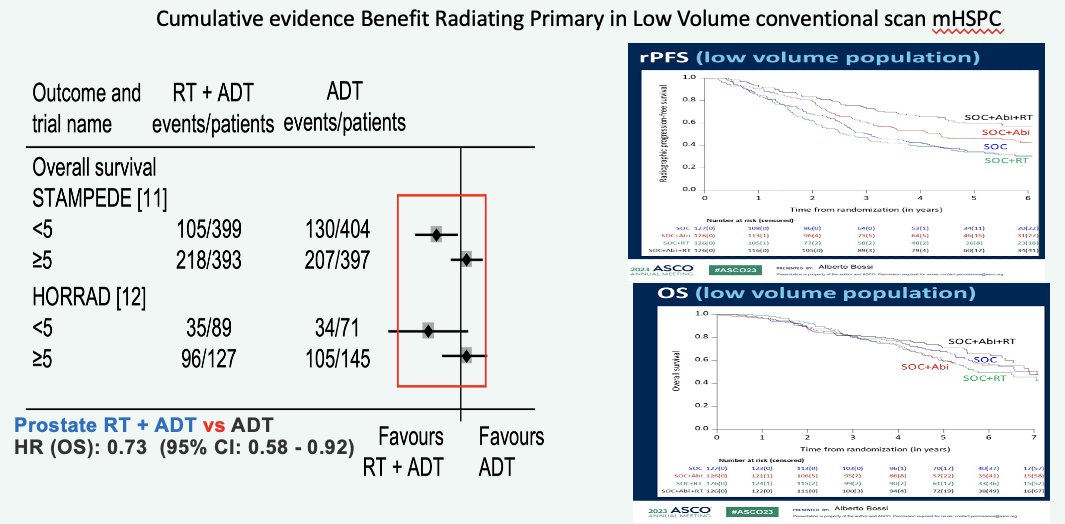
Now that we have established a likely benefit for prostate radiotherapy for PSMA-detected M1a patients, what is the optimal systemic therapy regimen? It appears that the answer is ADT plus an androgen receptor pathway inhibitor. Data from the CHAARTED trial of ADT +/- docetaxel for metastatic hormone sensitive prostate cancer patients and the 2023 STOPCaP individual patient data meta-analysis of CHAARTED, GETUG-15, and STAMPEDE-docetaxel suggest that there is less of a docetaxel benefit in synchronous, low volume metastatic hormone sensitive patients.5,6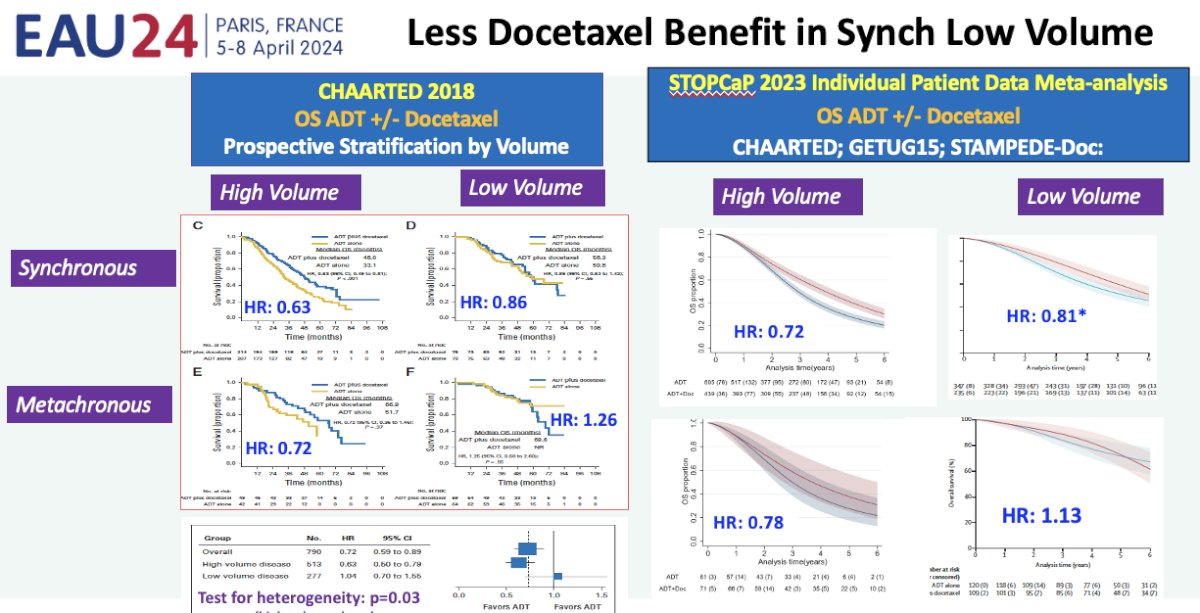
Additionally, a living network meta-analysis presented at ASCO GU 2024 indirectly demonstrated that there was no evidence that adding docetaxel to a doublet regimen of ADT + an androgen receptor pathway inhibitor will benefit patients with low-volume disease on conventional imaging. By simple extrapolation, one can deduce that this would likely include patients with PSMA-detected M1a disease, thus reinforcing that such patients are likely best served with doublet systemic therapy approaches.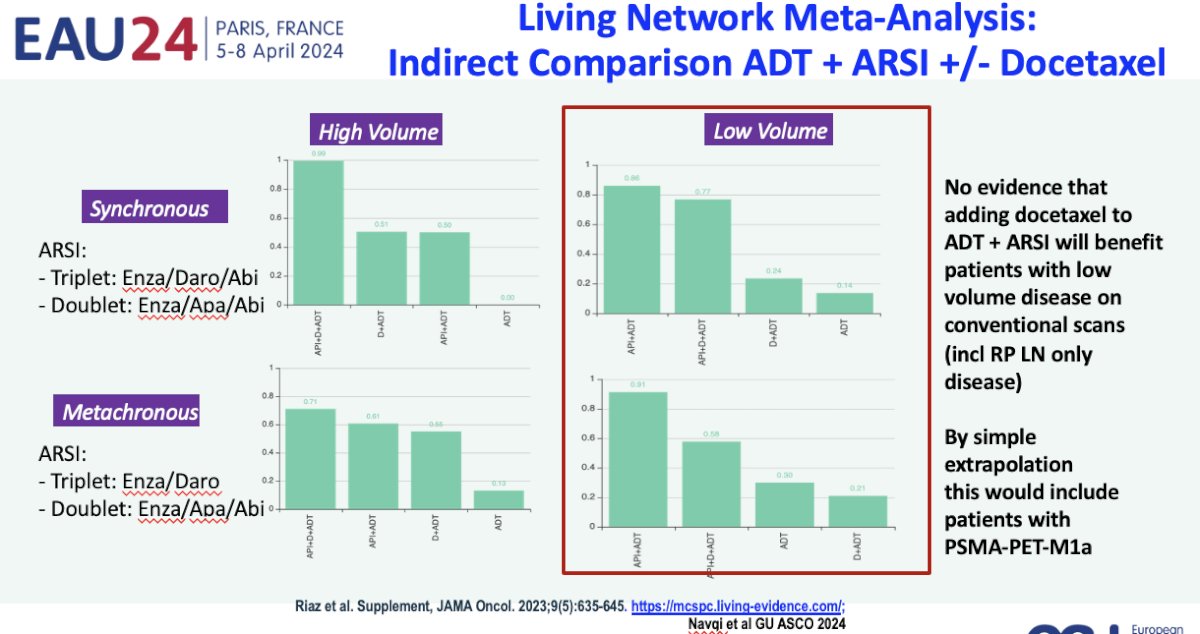
Dr. Sweeney concluded by re-emphasizing that patients with de novo PSMA-detected M1a disease should be treated similar to those with conventional imaging-detected M1 disease with ADT + an androgen receptor pathway inhibitor. As for the patient who he presented at the start who has de novo PSMA-PET/CT M1a disease, but conventional scan negative disease (i.e., CT < 1.0 cm) who requests “Hit me with your best shot!”, he proposes that ADT + an androgen receptor pathway inhibitor + prostate radiotherapy is the ideal approach for this patient.
Presented by: Professor Christopher Sweeney, MBBS, Medical Oncology, South Australian Immunogenomics Cancer Institute, University of Adelaide, Adelaide, South Australia, Australia
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomized controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 2022 Jan 29;399(10323):447-460.
- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019 Mar;75(3):410-418.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE
- Burdett S, Boeve LM, Ingleby FC, et al. Prostate radiotherapy for metastatic hormone-sensitive prostate cancer: A STOPCAP Systematic Review and Meta-analysis. Eur Urol. 2019 Jul;76(1):115-124.
- Gravis G, Boher JM, Chen YH, et al. Burden of Metastatic Castrate Naïve Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur Urol 2018 Jun;73(6):847-855.
- Vale CL, Fisher DJ, Godolphin PJ, et al. Which patients with metastatic hormone sensitive prostate cancer benefit from docetaxel: A systematic review and meta-analysis of individual participant data from randomized trials. Lancet Oncol. 2023 Jul(7):783-797.


