(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Professor Ken Herrmann discussed the advantages and pitfalls of molecular imaging for prostate cancer characterization.
There are currently three Food and Drug Administration (FDA)-approved positron emission tomography (PET) tracers for prostate cancer:
- 68Ga-PSMA-11
- 18 F-DCFPyL
- 18F-rh-PSMA-7.3

What are the studies that have led to the approvals for the PET traces for primary staging of intermediate-high risk prostate cancer?
In 2021, Hope et al. published results of a multicenter, single-arm, open-label phase 3 trial evaluating 68Ga-PSMA-11 PET/CT for the detection of pelvic nodal metastases in patients with intermediate and high-risk prostate cancer (at least one of the following: PSA >10 ng/mL, ≥cT2b, Gleason Score ≥7). The reference standard was histopathology obtained at the time of pelvic lymph node dissection. Pathologic nodal involvement was present in 75/277 patients (27%). The sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases were 40% (95% CI: 34 – 46%), 96% (95% CI: 92 – 97%), 75% (95% CI: 70 – 80%), and 81% (95% CI: 76 – 85%), respectively. The limitations of PSMA PET/CT for detecting micrometastatic deposits were highlighted with true-positive lymph nodes detected having a mean size of 1.1 cm, compared to 0.6 cm for false-negative lymph nodes.1
ProPSMA was a multi-center, two-arm randomized controlled trial of men with histologically confirmed prostate cancer who were being considered for curative intent radical prostatectomy or radiotherapy. To be eligible for inclusion, men must have had at least one high-risk factor including PSA ≥ 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging consisting of bone scan and CT or 68Ga-PSMA-11 PET/CT. The primary study outcome was the accuracy of first-line diagnostic imaging for identifying either pelvic nodal or distant metastatic disease. Accuracy was reported using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. The reference standard was a composite panel of histopathology, imaging, and biochemistry at 6-month follow-up.
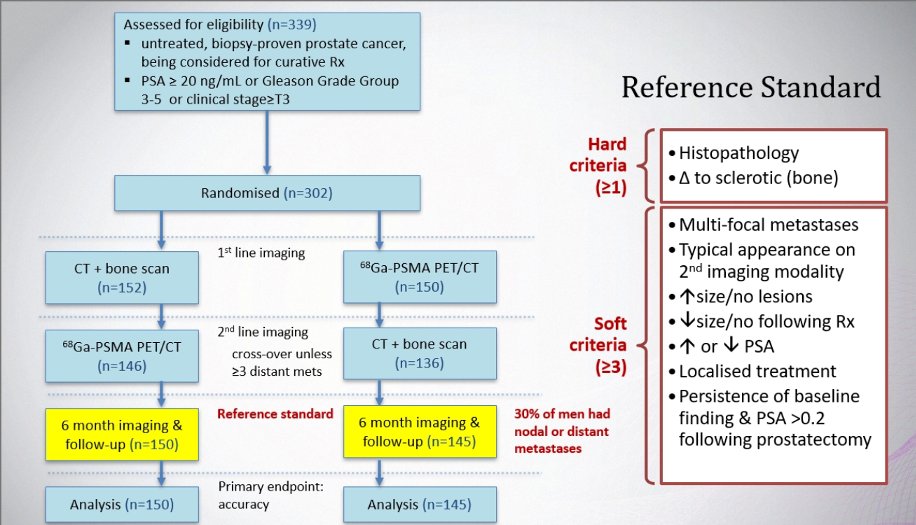
Between 2017 and 2018, 302 patients were randomized to either conventional imaging (n = 152) or 68Ga-PSMA-11 PET/CT (n = 150). As planned, the study cohort exhibited high-risk disease features: 293 (97%) men had ISUP grade 3 or higher, 65 (21.5%) had PSA 20 ng/mL or higher, and 82 (27.2%) had clinical stage T3 or T4 disease. With regards to the primary outcome, PSMA PET/CT had a 27% absolute greater AUC for accuracy compared to conventional imaging (95% CI for difference: 23 – 31%): 92% (95% CI: 88 – 95%) versus 65% (95% CI: 60 – 69%):
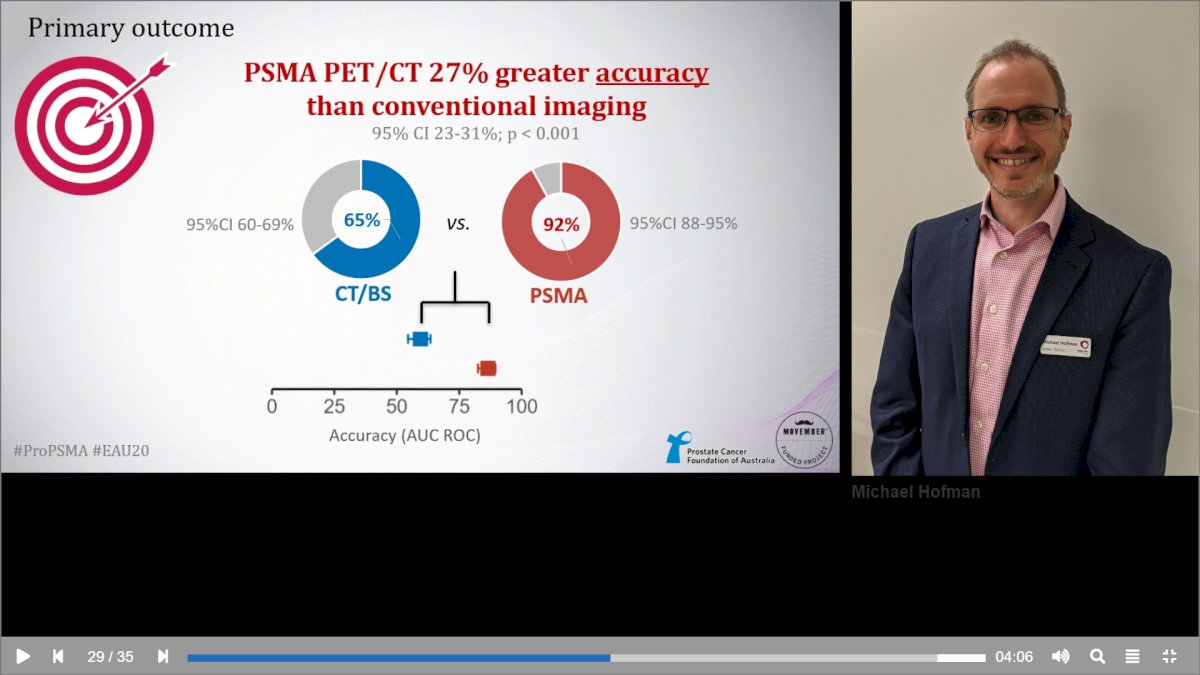
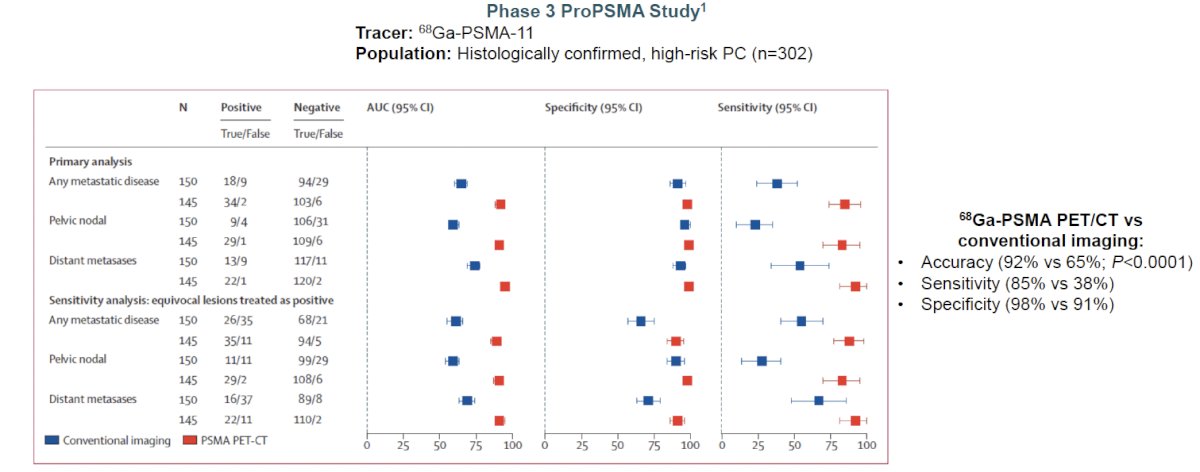
Conventional imaging had both a lower sensitivity (38% vs. 85%) and specificity (91% vs. 98%). Subgroup analyses by site of metastasis demonstrated the superiority of PSMA PET/CT for pelvic nodal (AUC: 91% versus 59%) and distant metastases (AUC: 95% versus 74%).2
The next tracer to be evaluated in this setting was 18F-DCFPyL. In 2021, the results of the Observe Prostate Cancer with Accuracy (OSPREY) trial were published. This was a prospective, multicenter, multi-reader, open-label phase 2/3 trial that included two distinct cohorts:
- Cohort A: Men with newly diagnosed high-risk prostate cancer (i.e., clinical stage ≥T3a or PSA >20 ng/ml or Gleason score ≥8) planned for radical prostatectomy with lymph node dissection
- Cohort B: Men with presumptive radiological evidence of recurrent or metastatic prostate cancer on conventional imaging and in whom lesions were considered amenable to biopsy
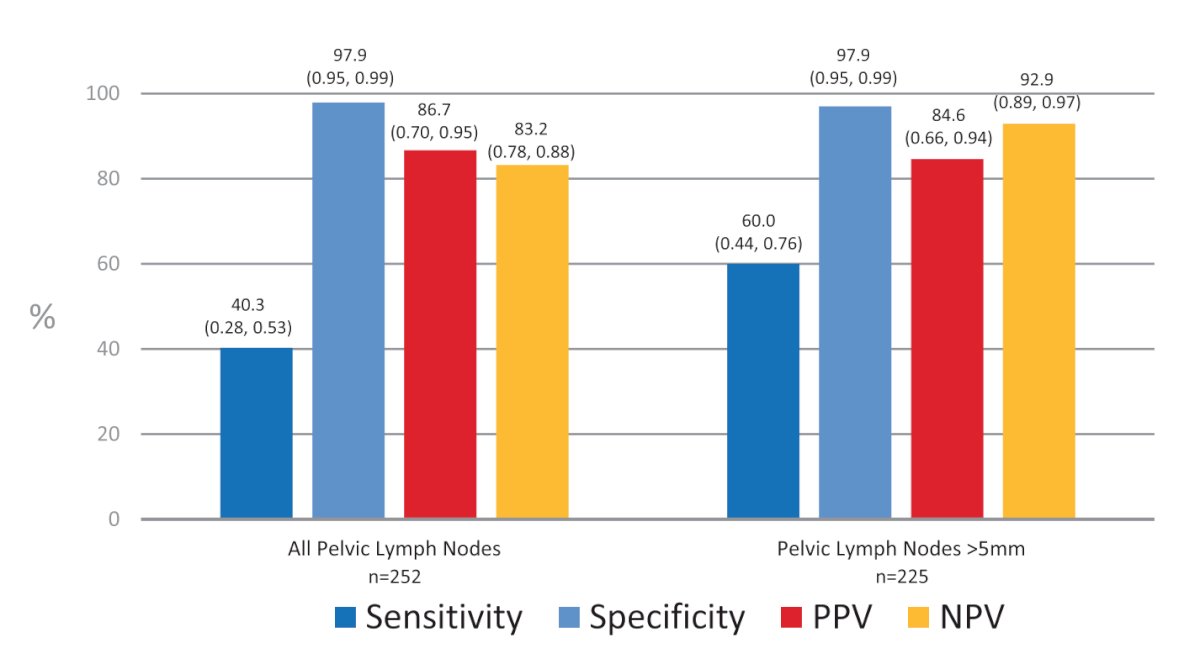
All patients underwent whole-body bone imaging and contrast-enhanced CT imaging 4-6 weeks prior to 18F-DCFPyL-PET/CT. Importantly, images were independently evaluated by three blinded nuclear medicine physicians. For cohort A, the reference standard was histopathologic analysis of the lymph nodes obtained at time of the extended template dissection (external and internal iliac, obturator fossa). Cohort A included 268 patients, with 252 having evaluable histopathology. The sensitivity and specificity of 18F-DCFPyL-PET/CT were 40.3% (95% CI: 28 – 53%) and 97.9% (95% CI: 95 – 99%), respectively. The positive and negative predictive values were 86.7% (95% CI: 70 – 95%) and 83.2% (95% CI: 78 – 88%), respectively. Primary tumor in the prostate was identified by the blinded readers in 95.2 – 99.3% of cases. Compared to conventional CT, 18F-DCFPyL-PET/CT demonstrated higher specificity (97.9% versus 65.1%), more than threefold higher positive predictive value (86.7% versus 28.3%), higher specificity (97.9% versus 65.1%), and similar sensitivity (40.3% versus 42.6%).3
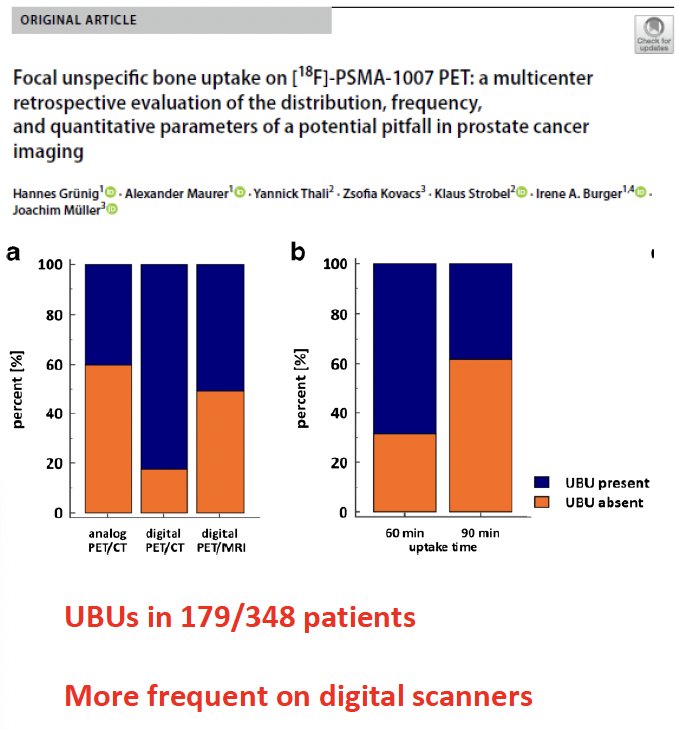
What are the pitfalls and challenges of PSMA-PET/CT in the primary staging setting? There appears to be an increased frequency of unspecific bone uptake, particularly when 18F-PSMA-1007 is used. In 2021, Grunig et al. demonstrated that unspecific bone uptake, defined as focal mild-to-moderate uptake (SUVmax < 10.0) not obviously related to a benign or malignant cause, was present in 51.4% of patients undergoing [18F]-PSMA-1007 PET scans.4 There appear to be inter-tracer differences in the rate of these false positive lesions, with Dr. Herrmann’s group demonstrating that such lesions are more than twice as frequent with 18F-PSMA-1007 PET, compared to 68Ga-PSMA-11 (140 versus 64, p<0.001).5
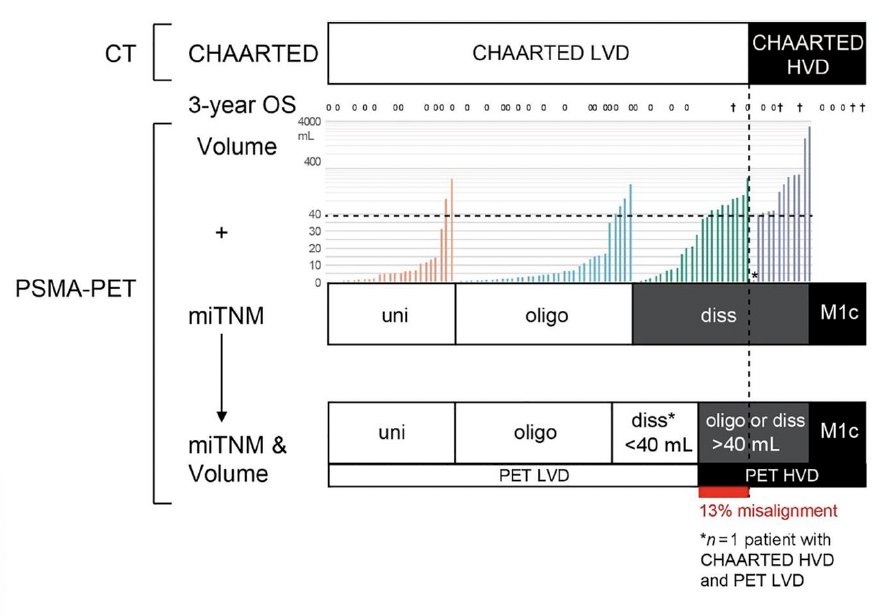
Another important challenge with the use of PSMA PET imaging is that current treatment recommendations are based on conventional imaging findings. As such, there have been increased efforts to ‘bridge the gap’ between conventional and PET imaging findings. In 2021, Barbato et al. demonstrated that a PSMA-positive tumor volume of 40 ml is the optimal cut-off to use when differentiating between CT-defined CHAARTED low- and high-volume disease.6
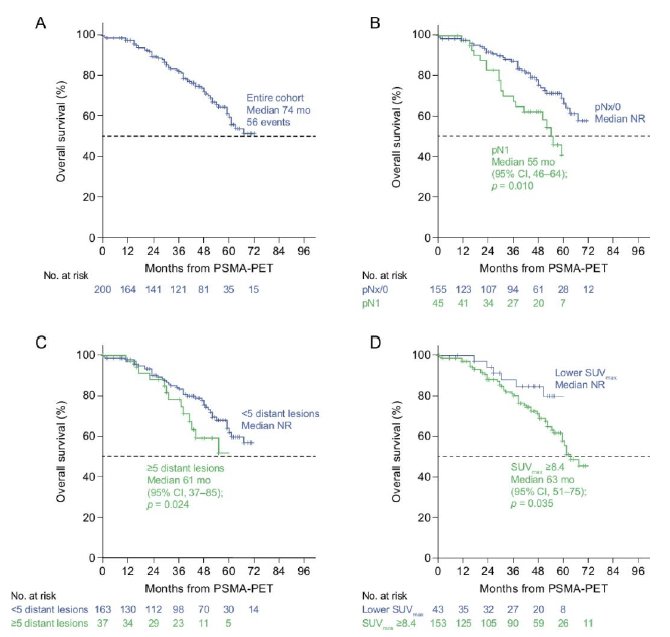
Additionally, there have been efforts to translate PET/CT findings to clinical outcomes, particularly among patients with non-metastatic castration-resistant prostate cancer (nmCRPC). It has previously been demonstrated that approximately 55% of patients with conventional imaging-defined nmCRPC have evidence of metastatic disease on PSMA-PET/CT. In a follow-up analysis, Weber and colleagues demonstrated that polymetastatic disease (≥5 distant lesions on PET) was independently associated with:
- Shorter overall survival (HR: 1.81, p=0.05)
- Time to new metastases (HR: 1.80, p=0.019)
These important data provide additional risk stratification for patients with nmCRPC and prognosticate their outcomes.7
There have been concerted efforts to standardize the analysis and reporting of PSMA-PET/CT with the SPARC (Standardized PSMA PET Analysis and Reporting Consensus) project currently underway, led by Professor Anders Bjartell.
The next step/challenge is to incorporate molecular imaging into clinical trials. Notable trials in this space include PRIMORDIUM (NCT04557059), which is evaluating the addition of apalutamide to radiotherapy and an LHRH agonist in high-risk patients with PSMA-PET-positive hormone-sensitive prostate cancer, and ARASTEP (NCT05794906), which is evaluating darolutamide plus ADT in patients with high-risk biochemically recurrent prostate cancer.

Professor Herrmann concluded with the following:
- PSMA PET is the best imaging test available for staging prostate cancer patients
- Pitfalls can be addressed by training and additional data
- Standardization is needed to secure the long-term success of PSMA PET
- SPARC is attempting to build a framework and host all existing classifications/stakeholders
- It is important to implement PET in all future clinical trials
Presented by: Professor Ken Herrmann, MD, Department of Nuclear Medicine, University Hospital Essen, Essen, Germany
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024
References:
- Hope TA, Eiber M, Armstrong WR, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021;7(11): 1635-42.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231): 1208-1216.
- Pienta KJ, Gorin MA, Rowe SP, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18 F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol 2021;206(1): 52-61.
- Grunig H, Maurer A, Thali Y, et al. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: a multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur J Nucl Med Mol Imaging. 2021;48(13): 4483-4494.
- Seifert R, Telli T, Opitz M, et al. Unspecific 18F-PSMA-1007 Bone Uptake Evaluated Through PSMA-11 PET, Bone Scanning, and MRI Triple Validation in Patients with Biochemical Recurrence of Prostate Cancer. J Nucl Med. 2023;64(5): 738-743.
- Barbato F, Fendler WP, Rauscher I, et al. PSMA-PET for the assessment of metastatic hormone-sensitive prostate cancer volume of disease. J Nucl Med. 2021;62(12): 1747-1750.
- Weber M, Fendler WP, Kumar ASR, et al. Prostate-specific Membrane Antigen Positron Emission Tomography-detected Disease Extent and Overall Survival of Patients with High-risk Nonmetastatic Castration-resistant Prostate Cancer: An International Multicenter Retrospective Study. Eur Urol. 2024: S0302-2838(24)00054-X


