(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Professor Anders Bjartell presented the SPARC (Standardized PSMA PET Analysis and Reporting Consensus) initiative.
There is a plethora of evidence supporting the use of PSMA-PET/CT for the staging of patients both in the initial and biochemically recurrent settings.

Additionally, we have witnessed the emergence of PSMA as a theranostics target with two notable trials in the metastatic castrate-resistant prostate cancer (mCRPC) space (VISION and TheraP) leading to the regulatory approval of 177Lu-PSMA-617 in the post-chemotherapy/androgen receptor pathway inhibitor setting.1,2
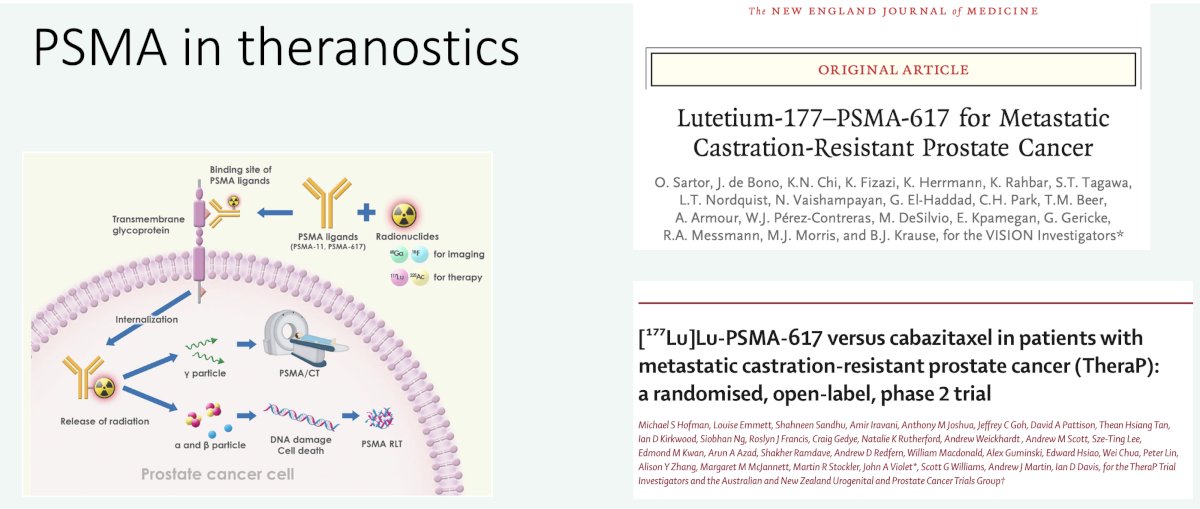
Clearly, the ‘train has left the station’ and there is a need to have clear and standardized communications between nuclear medicine physicians and clinicians.

There have been numerous efforts to standardize the reporting of PSMA-PET/CT. There are currently two EAU-EANM (European Association of Nuclear Medicine) consensus statements on PSMA-PET/CT relating to both response assessment criteria and the use of 177Lu-PSMA radioligand therapy.

Additional efforts in this space have included the EANM Focus 5 consensus meeting on PSMA-PET/CT which was published in 2023.3

In conjunction with the already published and still ongoing research of using PSMA PET beyond the currently guideline-recommended applications, numerous classifications have also been established to standardize the reporting of PSMA PET. The range of introduced classifications includes:
- Primary diagnosis (e.g., PRIMARY)
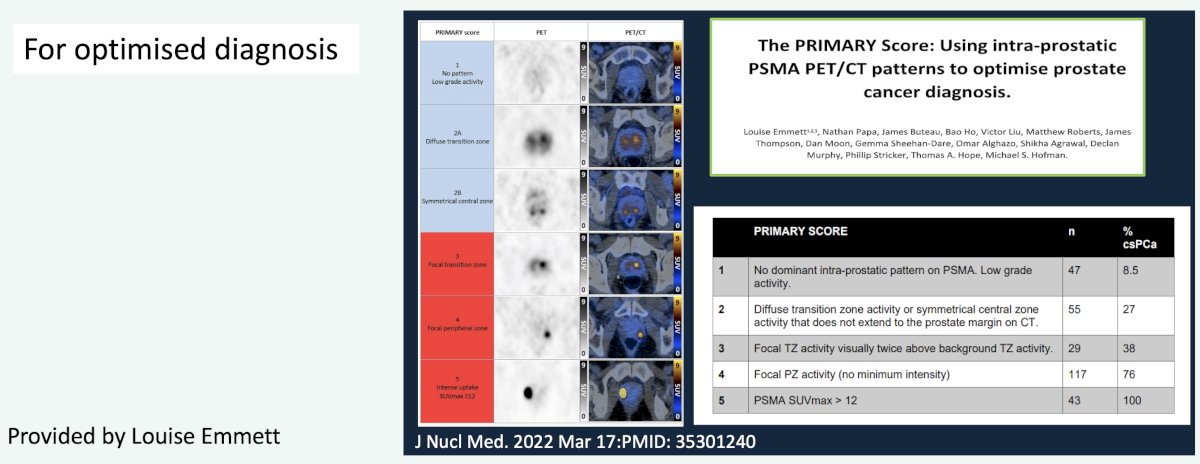
- General PSMA PET reporting recommendations (e.g., PROMISE, PSMA-RADS)

- Therapy response assessment (e.g. PPP, e-PSMA, PCWG3 [not including PSMA PET yet], or RECIP for PSMA radioligand therapy).

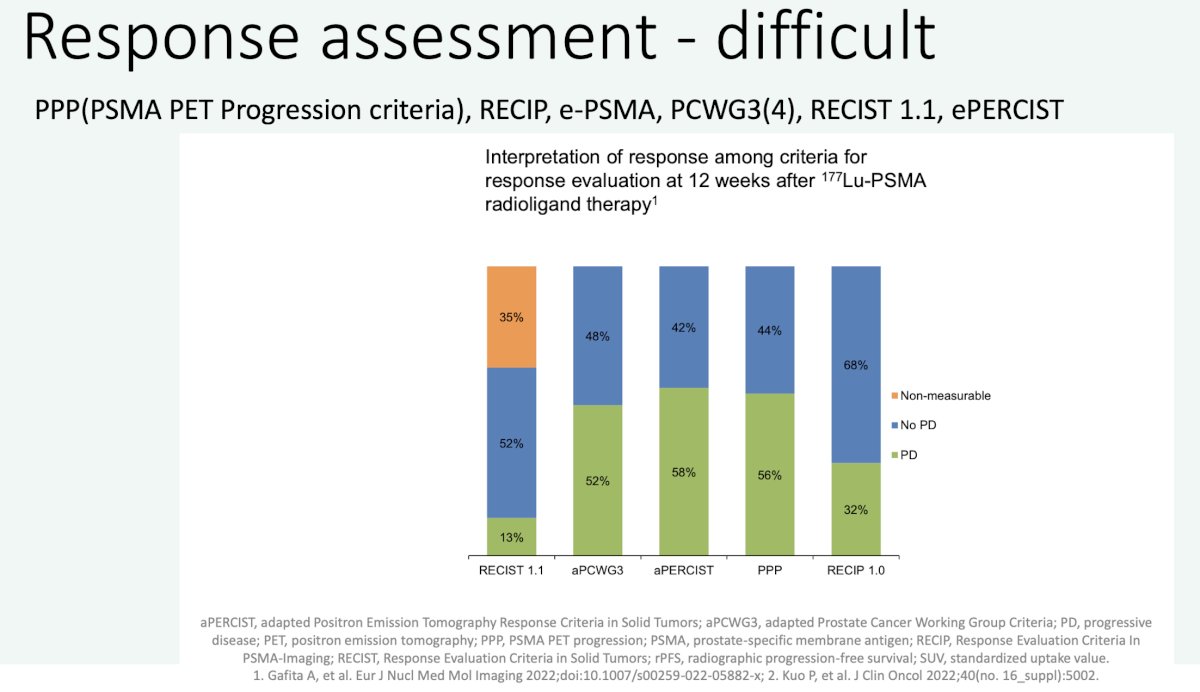
The SPARC project intends to initiate a process of combining classifications and recommendations under one unifying umbrella to establish a living framework of PSMA PET reporting. Accordingly, it is intended to bring all involved stakeholders including academic societies, industry, and active individuals together. The design for SPARC is summarized below:
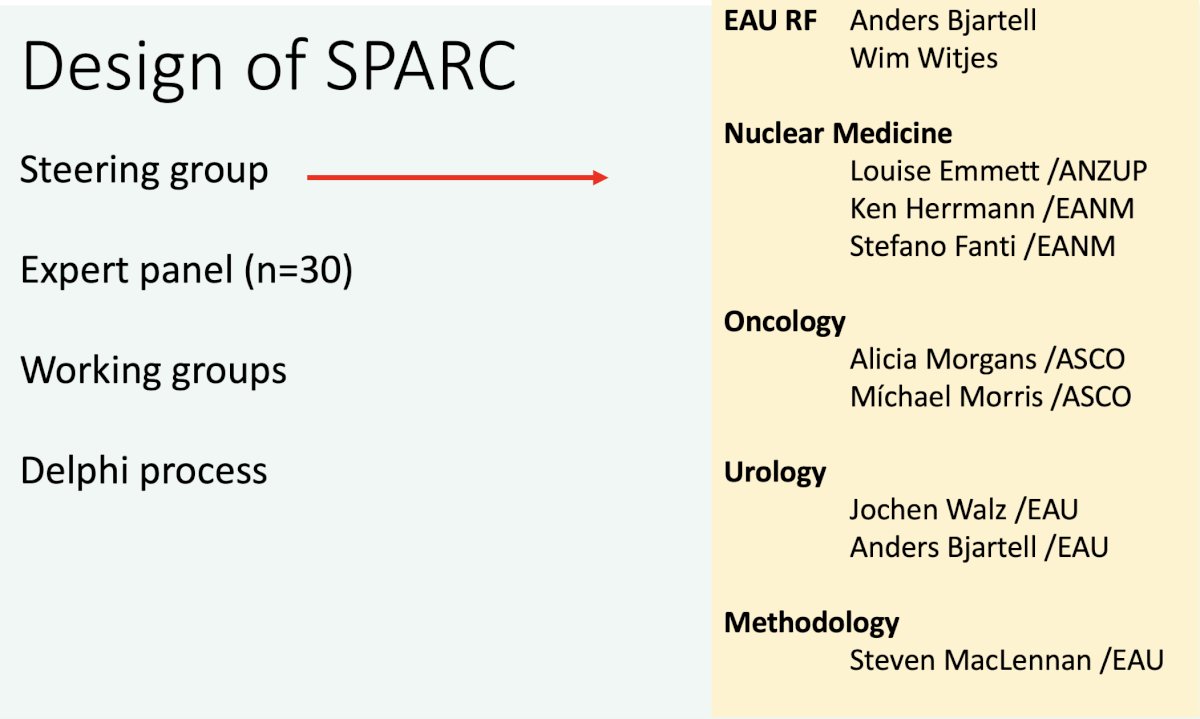
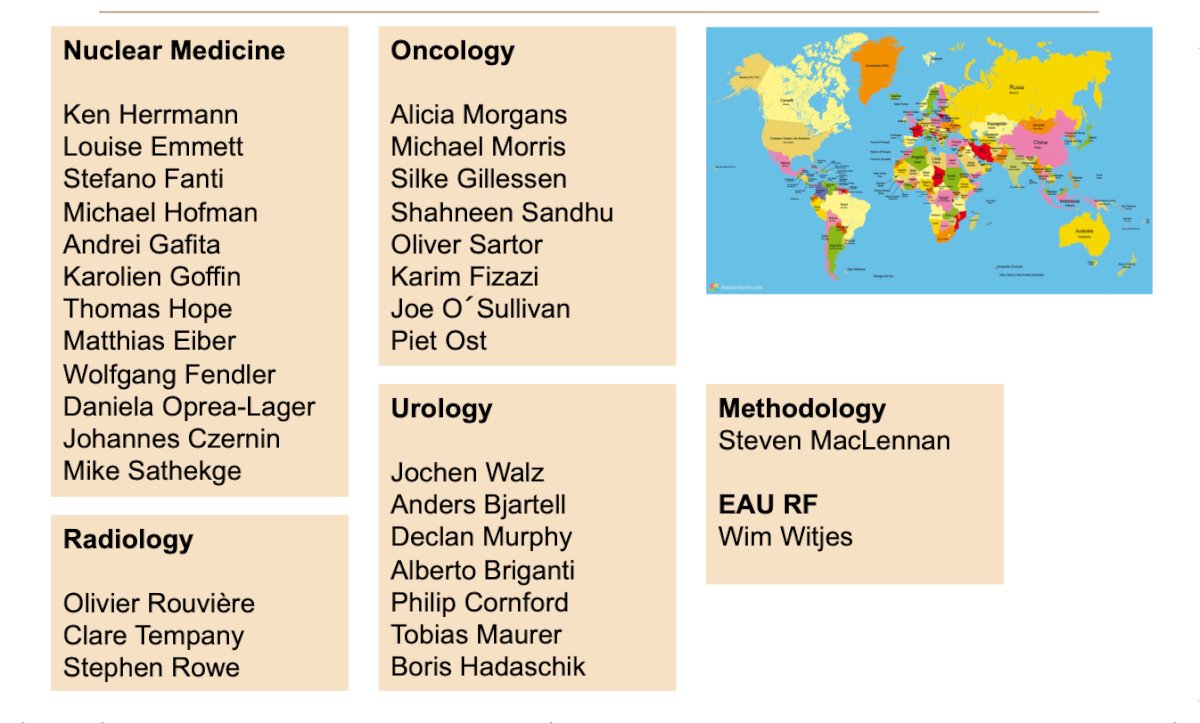
The SPARC panel includes experts from the following disciplines:
The SPARC working groups are organized/assigned as follows:

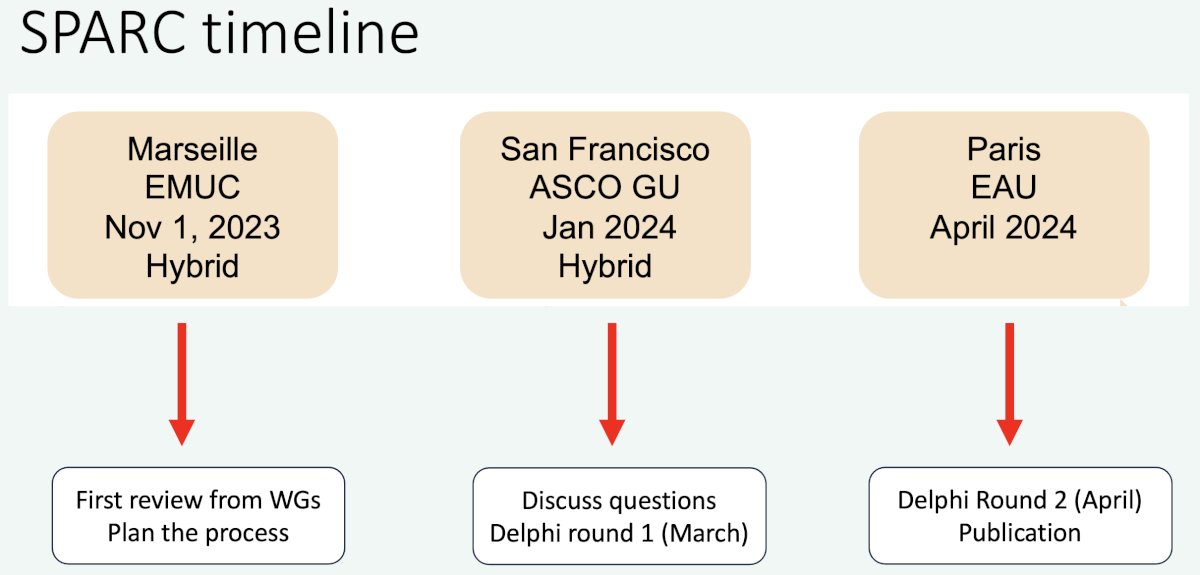
The SPARC timeline is summarized below:
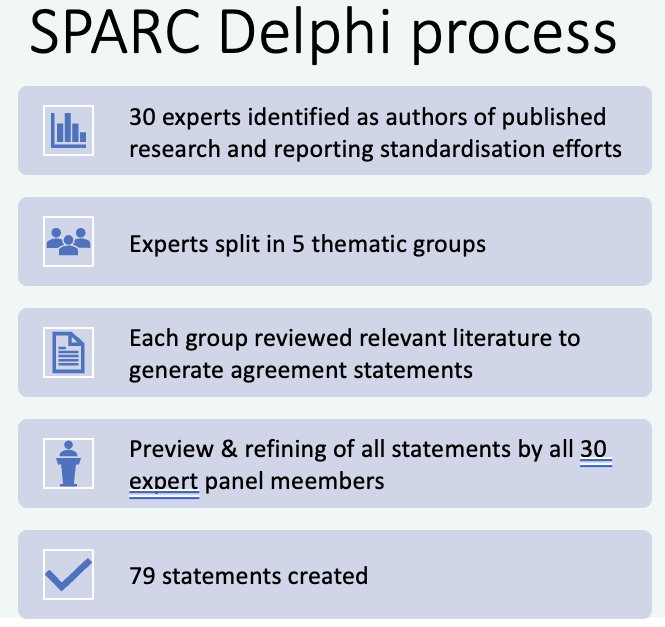
The SPARC Delphi process is being conducted as follows:
- All statements are scored in Delphi round 1 on a 1 – 9 agreement scale
- In round 2, experts are reminded of their own score and are shown a summary of other’s scores, then re-vote
- An analysis using RAND appropriateness method (median and interpercentile range based) is subsequently performed
- A final meeting to re-vote on any statements not meeting consensus via Delphi is performed
Presented by: Professor Anders Bjartell, MD, PhD, Professor in Urology, Skåne University Hospital, Malmö, Sweden
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Oprea-Lager D, MacLennan S, Bjartell A, et al. European Association of Nuclear Medicine Focus 5: Consensus on Molecular Imaging and Theranostics in Prostate Cancer. Eur Urol. 2024;85(1): 49-60.


